CTLA4 depletes T cell endogenous and trogocytosed B7 ligands via cis-endocytosis
- PMID: 37042938
- PMCID: PMC10103642
- DOI: 10.1084/jem.20221391
CTLA4 depletes T cell endogenous and trogocytosed B7 ligands via cis-endocytosis
Abstract
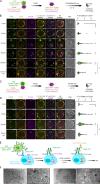
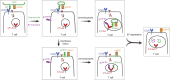
CD28 and CTLA4 are T cell coreceptors that competitively engage B7 ligands CD80 and CD86 to control adaptive immune responses. While the role of CTLA4 in restraining CD28 costimulatory signaling is well-established, the mechanism has remained unclear. Here, we report that human T cells acquire antigen-presenting-cell (APC)-derived B7 ligands and major histocompatibility complex (MHC) via trogocytosis through CD28:B7 binding. Acquired MHC and B7 enabled T cells to autostimulate, and this process was limited cell-intrinsically by CTLA4, which depletes B7 ligands trogocytosed or endogenously expressed by T cells through cis-endocytosis. Extending this model to the previously proposed extrinsic function of CTLA4 in human regulatory T cells (Treg), we show that blockade of either CD28 or CTLA4 attenuates Treg-mediated depletion of APC B7, indicating that trogocytosis and CTLA4-mediated cis-endocytosis work together to deplete B7 from APCs. Our study establishes CTLA4 as a cell-intrinsic molecular sink that limits B7 availability on the surface of T cells, with implications for CTLA4-targeted therapy.
© 2023 Xu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
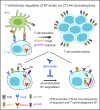
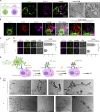







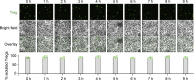

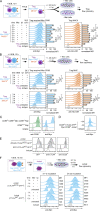


Comment in
-
CTLA4 prohibits T cells from cross-dressing.J Exp Med. 2023 Jul 3;220(7):e20230419. doi: 10.1084/jem.20230419. Epub 2023 Apr 18. J Exp Med. 2023. PMID: 37071124 Free PMC article.
References
-
- Akkaya, B., Oya Y., Akkaya M., Al Souz J., Holstein A.H., Kamenyeva O., Kabat J., Matsumura R., Dorward D.W., Glass D.D., and Shevach E.M.. 2019. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 20:218–231. 10.1038/s41590-018-0280-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

