Effect of Intrapleural Fibrinolytic Therapy vs Surgery for Complicated Pleural Infections: A Randomized Clinical Trial
- PMID: 37043201
- PMCID: PMC10098968
- DOI: 10.1001/jamanetworkopen.2023.7799
Effect of Intrapleural Fibrinolytic Therapy vs Surgery for Complicated Pleural Infections: A Randomized Clinical Trial
Abstract
Importance: There is a paucity of high-quality prospective randomized clinical trials comparing intrapleural fibrinolytic therapy (IPFT) with surgical decortication in patients with complicated pleural infections.
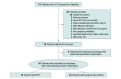
Objective: To assess the feasibility, safety, and efficacy of an algorithm comparing tissue plasminogen activator plus deoxyribonuclease therapy with surgical decortication in patients with complicated pleural infections.
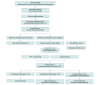
Design, setting, and participants: This parallel pilot randomized clinical trial was performed at a single urban community-based center from March 1, 2019, to December 31, 2021, with follow-up for 90 days. Seventy-four individuals were screened and 48 were excluded. Twenty-six patients 18 years or older with clinical pleural infection and positive findings of pleural fluid analysis were included. Of these, 20 patients underwent randomized selection (10 in each group), and 6 were observed.
Interventions: Intrapleural tissue plasminogen activator plus deoxyribonuclease therapy vs surgical decortication.
Main outcomes and measures: Primary outcomes were the percentage of patients enrolled to study completion and multidisciplinary adherence. Secondary outcomes included the number of patients with and the reason for inadequate screening, screening to enrollment failures, time to accrual of 20 patients or the number accrued at 1 year, and clinical data.
Results: Twenty-six patients were enrolled, 10 were randomized to each group, and 6 were observed. There was 100% enrollment to study completion in each treatment group, no protocol deviations, 2 minor protocol amendments, and no screening to enrollment failures. It took 32 months to enroll 26 patients. The 20 randomized patients had a median age of 57 (IQR, 46-65) years, were predominantly men (15 [75%]), and had a median RAPID (Renal, Age, Purulence, Infection Source, and Dietary Factors) score of 2 (IQR, 1-3). Treatment failure occurred in 1 patient and 2 crossover treatments occurred, all of which were in the IPFT group. Intraprocedure and postprocedure complications were similar between the groups. There were no reoperations or in-hospital deaths. Median duration of chest tube use was comparable in the IPFT (5 [IQR, 4-8] days) and surgery (4 [IQR, 3-5] days) groups (P = .21). Median hospital stay tended to be longer in the IPFT (11 [IQR, 4-18] days) vs surgery (5 [IQR, 4-6] days) groups, although the difference as not significantly different (P = .08). There were no 30-day readmissions or 30- or 90-day deaths.
Conclusions and relevance: In this pilot randomized clinical trial, the study algorithm was feasible, safe, and efficacious. This provides evidence to move forward with a multicenter randomized clinical trial.
Trial registration: ClinicalTrials.gov Identifier: NCT03873766.
Conflict of interest statement
Figures
Similar articles
-
Intrapleural Fibrinolytic Therapy versus Early Medical Thoracoscopy for Treatment of Pleural Infection. Randomized Controlled Clinical Trial.Ann Am Thorac Soc. 2020 Aug;17(8):958-964. doi: 10.1513/AnnalsATS.202001-076OC. Ann Am Thorac Soc. 2020. PMID: 32421353 Clinical Trial.
-
Effectiveness of Intrapleural Tissue Plasminogen Activator and Dornase Alfa vs Tissue Plasminogen Activator Alone in Children with Pleural Empyema: A Randomized Clinical Trial.JAMA Pediatr. 2020 Apr 1;174(4):332-340. doi: 10.1001/jamapediatrics.2019.5863. JAMA Pediatr. 2020. PMID: 32011642 Free PMC article. Clinical Trial.
-
Intrapleural tissue plasminogen activator and deoxyribonuclease in complex pleural effusion and empyema, clinical outcomes, and predictors.Ther Adv Respir Dis. 2025 Jan-Dec;19:17534666251343711. doi: 10.1177/17534666251343711. Epub 2025 May 30. Ther Adv Respir Dis. 2025. PMID: 40444335 Free PMC article.
-
Intra-pleural fibrinolytic therapy versus placebo, or a different fibrinolytic agent, in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev. 2019 Oct 30;2019(10):CD002312. doi: 10.1002/14651858.CD002312.pub4. Cochrane Database Syst Rev. 2019. PMID: 31684683 Free PMC article.
-
Intrapleural agents for pleural infection: fibrinolytics and beyond.Curr Opin Pulm Med. 2012 Jul;18(4):326-32. doi: 10.1097/MCP.0b013e3283531149. Curr Opin Pulm Med. 2012. PMID: 22487944 Review.
Cited by
-
Early Video-assisted Thoracoscopic Surgery or Intrapleural Enzyme Therapy in Pleural Infection: A Feasibility Randomized Controlled Trial. The Third Multicenter Intrapleural Sepsis Trial-MIST-3.Am J Respir Crit Care Med. 2023 Dec 15;208(12):1305-1315. doi: 10.1164/rccm.202305-0854OC. Am J Respir Crit Care Med. 2023. PMID: 37820359 Free PMC article. Clinical Trial.
-
Medical thoracoscopy combined with intrapleural injection of urokinase for treatment of pleural infection-a multicenter, prospective, randomized controlled study: study protocol.Respir Res. 2025 Jan 18;26(1):21. doi: 10.1186/s12931-025-03106-y. Respir Res. 2025. PMID: 39827129 Free PMC article.
-
Surgery versus intrapleural fibrinolysis for management of complicated pleural infections: a systematic review and meta-analysis.Respir Res. 2024 Aug 24;25(1):323. doi: 10.1186/s12931-024-02949-1. Respir Res. 2024. PMID: 39182102 Free PMC article.
-
Enzymatic therapy versus video-assisted thoracoscopic surgery for pleural infections: a systematic review and meta-analysis.ERJ Open Res. 2025 Mar 3;11(2):00619-2024. doi: 10.1183/23120541.00619-2024. eCollection 2025 Mar. ERJ Open Res. 2025. PMID: 40040906 Free PMC article.
-
Ultrasound and Intrapleural Enzymatic Therapy for Complicated Pleural Effusion: A Case Series with a Literature Review.J Clin Med. 2024 Jul 25;13(15):4346. doi: 10.3390/jcm13154346. J Clin Med. 2024. PMID: 39124612 Free PMC article. Review.