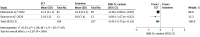Ketamine vs Electroconvulsive Therapy for Major Depressive Episode: A Systematic Review and Meta-analysis
- PMID: 37043224
- PMCID: PMC10099100
- DOI: 10.1001/jamapsychiatry.2023.0562
Ketamine vs Electroconvulsive Therapy for Major Depressive Episode: A Systematic Review and Meta-analysis
Erratum in
-
Errors in Figures.JAMA Psychiatry. 2023 Jun 1;80(6):651. doi: 10.1001/jamapsychiatry.2023.1752. JAMA Psychiatry. 2023. PMID: 37284883 Free PMC article. No abstract available.
Abstract
Importance: The relative efficacy of ketamine and electroconvulsive therapy (ECT) in adults with major depressive episode (MDE) needs clarification.
Objective: To compare depression rating outcomes with ketamine vs ECT in adults with MDE and to compare response and remission rates, number of sessions to response and remission, and adverse effects.
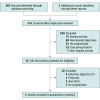
Data sources: Two investigators independently systematically searched MEDLINE, ScienceDirect, and Google Scholar databases using a combination of relevant Medical Subject Headings terms and free-text keywords from database inception through May 15, 2022, to identify relevant English-language trials.
Study selection: Parallel-group randomized clinical trials (RCTs).
Data extraction and synthesis: Two investigators independently extracted data and assessed risk of bias. One-week posttreatment outcomes were pooled as standardized mean difference (SMD; Hedges g) for continuous outcomes and risk ratio (RR) for categorical outcomes in random-effects meta-analyses.
Main outcomes and measures: Efficacy outcomes were 1-week (or nearest) posttreatment depression ratings, 1-week (or nearest) study-defined response and remission rates, and number of sessions to treatment response and remission. Safety outcomes were reported adverse effects.
Results: Five trials (ketamine group: n = 141; ECT group: n = 137) were meta-analyzed. The overall pooled SMD for posttreatment depression ratings was -0.39 (95% CI, -0.81 to 0.02; I2 = 45%; 5 RCTs). For this efficacy outcome, in a sensitivity analysis of methodologically stronger trials, ECT was superior to ketamine (SMD, -0.45; 95% CI, -0.75 to -0.14; I2 = 6%; 2 RCTs). ECT was also superior to ketamine for study-defined response (RR, 1.27; 95% CI, 1.06-1.53; I2 = 0%; 3 RCTs) and remission (RR, 1.43; 95% CI, 1.12-1.82; I2 = 0%; 2 RCTs) rates. No significant differences were noted between groups for number of sessions to response and remission and for cognitive outcomes. Key limitations were small number of studies, limited sample size, and high risk of bias in all trials.
Conclusion and relevance: The findings of this systematic review and meta-analysis suggest an efficacy advantage for ECT over ketamine in adults with MDE. These conclusions are tempered by the small number and size of existing trials.
Conflict of interest statement
Figures