Efficacy and Safety of Upadacitinib Treatment in Adolescents With Moderate-to-Severe Atopic Dermatitis: Analysis of the Measure Up 1, Measure Up 2, and AD Up Randomized Clinical Trials
- PMID: 37043227
- PMCID: PMC10099102
- DOI: 10.1001/jamadermatol.2023.0391
Efficacy and Safety of Upadacitinib Treatment in Adolescents With Moderate-to-Severe Atopic Dermatitis: Analysis of the Measure Up 1, Measure Up 2, and AD Up Randomized Clinical Trials
Erratum in
-
Errors in Abstract, Results, Tables, and Figures.JAMA Dermatol. 2024 May 1;160(5):582. doi: 10.1001/jamadermatol.2024.0241. JAMA Dermatol. 2024. PMID: 38506834 Free PMC article. No abstract available.
Abstract
Importance: Atopic dermatitis onset usually occurs in childhood. Persistence of disease into adolescence and adulthood is common. It is important to evaluate new treatment options in adolescents because of the high unmet need in this population.
Objective: To assess the efficacy and safety of upadacitinib to treat moderate-to-severe atopic dermatitis in adolescents.
Design, setting, and participants: Prespecified analysis of adolescents enrolled in 3 randomized, double-blind, placebo-controlled phase 3 clinical trials in more than 20 countries across Europe, North and South America, Oceania, the Middle East, and the Asia-Pacific region from July 2018 through December 2020. Participants were adolescents aged 12 to 17 years with moderate-to-severe atopic dermatitis. Data analysis was performed from April to August 2021.
Interventions: Patients were randomized (1:1:1) to once-daily oral upadacitinib 15 mg, upadacitinib 30 mg, or placebo alone (Measure Up 1 and Measure Up 2) or with topical corticosteroids (AD Up).
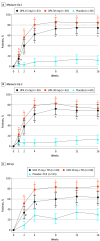
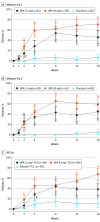
Main outcomes and measures: Safety and efficacy, including at least a 75% improvement in the Eczema Area and Severity Index from baseline and validated Investigator Global Assessment for Atopic Dermatitis score of 0 (clear) or 1 (almost clear) at week 16 (coprimary end points).
Results: A total of 552 adolescents (290 female; 262 male) were randomized. Mean (SD) age was 15.4 (1.8), 15.5 (1.7), and 15.3 (1.8) years for adolescents in Measure Up 1, Measure Up 2, and AD Up, respectively. In Measure Up 1, Measure Up 2, and AD Up, respectively, a greater proportion of adolescents (% [95% CI]) achieved at least 75% improvement in the Eczema Area and Severity Index at week 16 with upadacitinib 15 mg (73% [63%-84%], 69% [57%-81%], 63% [51%-76%]), and upadacitinib 30 mg (78% [68%-88%], 73% [62%-85%], 84% [75%-94%]), than with placebo (12% [4%-20%], 13% [5%-22%], 30% [19%-42%]; nominal P < .001 for all comparisons vs placebo). Similarly, a greater proportion of adolescents treated with upadacitinib achieved a validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1 at week 16 and improvements in quality of life with upadacitinib than with placebo. Upadacitinib was generally well tolerated in adolescents. Acne was the most common adverse event, and all acne events were mild or moderate.
Conclusions and relevance: In this analysis of 3 randomized clinical trials, upadacitinib was an effective treatment for adolescents with moderate-to-severe atopic dermatitis, with an acceptable safety profile.
Trial registration: ClinicalTrials.gov Identifiers: NCT03569293 (Measure Up 1), NCT03607422 (Measure Up 2), and NCT03568318 (AD Up).
Conflict of interest statement
Figures
Comment in
-
Reanalysis of Study After Removal of Participants in 1 Trial of Upadacitinib for Atopic Dermatitis.JAMA Dermatol. 2024 May 1;160(5):582. doi: 10.1001/jamadermatol.2024.0194. JAMA Dermatol. 2024. PMID: 38506791 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

