FXR1 regulates vascular smooth muscle cell cytoskeleton, VSMC contractility, and blood pressure by multiple mechanisms
- PMID: 37043351
- PMCID: PMC10564969
- DOI: 10.1016/j.celrep.2023.112381
FXR1 regulates vascular smooth muscle cell cytoskeleton, VSMC contractility, and blood pressure by multiple mechanisms
Abstract
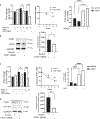
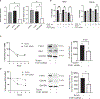
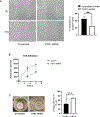
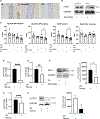
Appropriate cytoskeletal organization is essential for vascular smooth muscle cell (VSMC) conditions such as hypertension. This study identifies FXR1 as a key protein linking cytoskeletal dynamics with mRNA stability. RNA immunoprecipitation sequencing (RIP-seq) in human VSMCs identifies that FXR1 binds to mRNA associated with cytoskeletal dynamics, and FXR1 depletion decreases their mRNA stability. FXR1 binds and regulates actin polymerization. Mass spectrometry identifies that FXR1 interacts with cytoskeletal proteins, particularly Arp2, a protein crucial for VSMC contraction, and CYFIP1, a WASP family verprolin-homologous protein (WAVE) regulatory complex (WRC) protein that links mRNA processing with actin polymerization. Depletion of FXR1 decreases the cytoskeletal processes of adhesion, migration, contraction, and GTPase activation. Using telemetry, conditional FXR1SMC/SMC mice have decreased blood pressure and an abundance of cytoskeletal-associated transcripts. This indicates that FXR1 is a muscle-enhanced WRC modulatory protein that regulates VSMC cytoskeletal dynamics by regulation of cytoskeletal mRNA stability and actin polymerization and cytoskeletal protein-protein interactions, which can regulate blood pressure.
Keywords: CP: Cell biology; WRC proteins; blood pressure; cytoskeletal; mRNA stability; vascular smooth muscle cell.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no interests to disclose.
Figures



Similar articles
-
Genetic Deletion of FXR1 Reduces Intimal Hyperplasia and Induces Senescence in Vascular Smooth Muscle Cells.Am J Pathol. 2023 May;193(5):638-653. doi: 10.1016/j.ajpath.2023.01.006. Am J Pathol. 2023. PMID: 37080662 Free PMC article.
-
SM22α inhibits lamellipodium formation and migration via Ras-Arp2/3 signaling in synthetic VSMCs.Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C758-C767. doi: 10.1152/ajpcell.00033.2016. Epub 2016 Sep 14. Am J Physiol Cell Physiol. 2016. PMID: 27629412
-
FXR1 Is an IL-19-Responsive RNA-Binding Protein that Destabilizes Pro-inflammatory Transcripts in Vascular Smooth Muscle Cells.Cell Rep. 2018 Jul 31;24(5):1176-1189. doi: 10.1016/j.celrep.2018.07.002. Cell Rep. 2018. PMID: 30067974 Free PMC article.
-
[The role of SM22 alpha in cytoskeleton organization and vascular remodeling].Sheng Li Ke Xue Jin Zhan. 2006 Jul;37(3):211-5. Sheng Li Ke Xue Jin Zhan. 2006. PMID: 17009727 Review. Chinese.
-
Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung.Respir Physiol Neurobiol. 2003 Sep 16;137(2-3):151-68. doi: 10.1016/s1569-9048(03)00144-7. Respir Physiol Neurobiol. 2003. PMID: 14516723 Review.
Cited by
-
Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage.Int J Mol Sci. 2025 Aug 6;26(15):7606. doi: 10.3390/ijms26157606. Int J Mol Sci. 2025. PMID: 40806733 Free PMC article. Review.
-
The FXR1 network acts as a signaling scaffold for actomyosin remodeling.Cell. 2024 Sep 5;187(18):5048-5063.e25. doi: 10.1016/j.cell.2024.07.015. Epub 2024 Aug 5. Cell. 2024. PMID: 39106863
-
The Spatial-Temporal Alternative Splicing Profile Reveals the Functional Diversity of FXR1 Isoforms in Myogenesis.Adv Sci (Weinh). 2024 Dec;11(47):e2405157. doi: 10.1002/advs.202405157. Epub 2024 Nov 5. Adv Sci (Weinh). 2024. PMID: 39499773 Free PMC article.
-
The FXR1 network acts as signaling scaffold for actomyosin remodeling.bioRxiv [Preprint]. 2024 May 25:2023.11.05.565677. doi: 10.1101/2023.11.05.565677. bioRxiv. 2024. Update in: Cell. 2024 Sep 5;187(18):5048-5063.e25. doi: 10.1016/j.cell.2024.07.015. PMID: 37961296 Free PMC article. Updated. Preprint.
References
-
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, and Nakano T (2003). Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 92, 411–418. 10.1161/01.RES.0000059987.90200.44. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

