Epidemiology of strongyloidiasis determined by parasite-specific IgG detections by enzyme-linked immunosorbent assay on urine samples using Strongyloides stercoralis, S. ratti and recombinant protein (NIE) as antigens in Northeast Thailand
- PMID: 37043507
- PMCID: PMC10096234
- DOI: 10.1371/journal.pone.0284305
Epidemiology of strongyloidiasis determined by parasite-specific IgG detections by enzyme-linked immunosorbent assay on urine samples using Strongyloides stercoralis, S. ratti and recombinant protein (NIE) as antigens in Northeast Thailand
Abstract
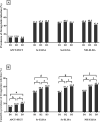
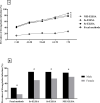
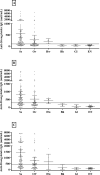
Detection of anti-Strongyloides IgG in urine by enzyme-linked immunosorbent assay (ELISA) for diagnosis of strongyloidiasis reportedly has comparable performance to conventional serum assays. Initial comparisons of urine assays using commercial ELISA kits designated for serology have shown its diagnostic potential but sub-optimal accuracy. In the present study, we optimized urine ELISA protocols based on different antigen types and evaluated their accuracies in determining the epidemiology of strongyloidiasis in Northeast Thailand. Paired urine and fecal samples of 966 individuals from the study community were collected for three consecutive days and tested for strongyloidiasis. We compared three ELISA protocols using different antigens including crude S. stercoralis antigen (Ss-ELISA), crude S. ratti antigen (Sr-ELISA) and recombinant NIE antigen (NIE-ELISA) and fecal examination by agar plate-culture (APCT) technique and formalin-ethyl acetate concentration technique (FECT). The optimized ELISA protocols using three different antigen sources yielded significantly higher prevalence rates of strongyloidiasis (58.9-65.1%) than those by fecal examination methods (19.7%). The prevalence of strongyloidiasis determined by ELISA protocols significantly increased with age (p value < 0.0001) and males had higher prevalence than females (p value < 0.0001). Diagnostic agreements between ELISA protocols were moderate (κ = 0.461-0.586) and the agreement between each ELISA protocol and fecal examinations were slight (κ = 0.139-0.210). The results obtained by urine ELISA protocols using three different antigens showed comparable diagnostic performances, provided further supports for the utility of urine as an alternative clinical specimen for diagnosis of strongyloidiasis.
Copyright: © 2023 Eamudomkarn et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Partially purified Strongyloides ratti antigen improved the diagnostic performance of strongyloidiasis by enzyme-linked immunosorbent assay (ELISA) and immunochromatographic test (ICT).Microbiol Spectr. 2025 Mar 4;13(3):e0236824. doi: 10.1128/spectrum.02368-24. Epub 2025 Feb 4. Microbiol Spectr. 2025. PMID: 39902967 Free PMC article.
-
Analysis of Daily Variation for 3 and for 30 Days of Parasite-Specific IgG in Urine for Diagnosis of Strongyloidiasis by Enzyme-Linked Immunosorbent Assay.Acta Trop. 2021 Jun;218:105896. doi: 10.1016/j.actatropica.2021.105896. Epub 2021 Mar 20. Acta Trop. 2021. PMID: 33753029
-
Diagnostic accuracy of a novel enzyme-linked immunoassay for the detection of IgG and IgG4 against Strongyloides stercoralis based on the recombinant antigens NIE/SsIR.Parasit Vectors. 2021 Aug 18;14(1):412. doi: 10.1186/s13071-021-04916-x. Parasit Vectors. 2021. PMID: 34407876 Free PMC article.
-
Screening of Strongyloides infection using an ELISA test in transplant candidates.Clinics (Sao Paulo). 2019;74:e698. doi: 10.6061/clinics/2019/e698. Epub 2019 Jun 6. Clinics (Sao Paulo). 2019. PMID: 31188909 Free PMC article. Review.
-
Strongyloidiasis: an emerging infectious disease in China.Am J Trop Med Hyg. 2013 Mar;88(3):420-5. doi: 10.4269/ajtmh.12-0596. Am J Trop Med Hyg. 2013. PMID: 23468357 Free PMC article. Review.
Cited by
-
Prevalence of asymptomatic strongyloidiasis co-infection in COVID-19 patients residing in endemic areas.Eur J Med Res. 2023 Aug 11;28(1):281. doi: 10.1186/s40001-023-01262-9. Eur J Med Res. 2023. PMID: 37563592 Free PMC article.
-
Partially purified Strongyloides ratti antigen improved the diagnostic performance of strongyloidiasis by enzyme-linked immunosorbent assay (ELISA) and immunochromatographic test (ICT).Microbiol Spectr. 2025 Mar 4;13(3):e0236824. doi: 10.1128/spectrum.02368-24. Epub 2025 Feb 4. Microbiol Spectr. 2025. PMID: 39902967 Free PMC article.
-
Examination of Diagnostic Performance of New IgG4 Rapid Test Compared with IgG- and IgG4-ELISAs to Investigate Epidemiology of Strongyloidiasis in Northeast Thailand.Am J Trop Med Hyg. 2024 Jan 9;110(2):254-262. doi: 10.4269/ajtmh.23-0518. Print 2024 Feb 7. Am J Trop Med Hyg. 2024. PMID: 38190756 Free PMC article.
-
Systematic Review of Strongyloides stercoralis Infection Diagnosis in Southeast Asia: Insights from Parasitological, Molecular, and Serological Approaches.Am J Trop Med Hyg. 2024 Aug 13;111(4):724-735. doi: 10.4269/ajtmh.23-0599. Print 2024 Oct 2. Am J Trop Med Hyg. 2024. PMID: 39137756
-
Diagnostic value of urinary and serum IgG antibodies in evaluating drug treatment response in strongyloidiasis assessed by fecal examination and digital droplet PCR.PLoS One. 2024 Dec 3;19(12):e0306732. doi: 10.1371/journal.pone.0306732. eCollection 2024. PLoS One. 2024. PMID: 39625913 Free PMC article.
References
-
- Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, et al.. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7(5):e2165. Epub 2013/05/16. doi: 10.1371/journal.pntd.0002165 ; PubMed Central PMCID: PMC3649958. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

