Contextual modifiers of healthspan, lifespan, and epigenome in mice under chronic social stress
- PMID: 37043532
- PMCID: PMC10120026
- DOI: 10.1073/pnas.2211755120
Contextual modifiers of healthspan, lifespan, and epigenome in mice under chronic social stress
Abstract
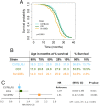
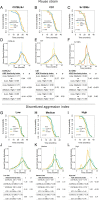
Sustained life stress and low socioeconomic status are among the major causes of aging-related diseases and decreased life expectancy. Experimental rodent models can help to identify the underlying mechanisms, yet very few studies address the long-term consequences of social stress on aging. We conducted a randomized study involving more than 300 male mice of commonly used laboratory strains (C57BL/6J, CD1, and Sv129Ev) chosen for the spontaneous aggression gradient and stress-vulnerability. Mice were exposed to a lifelong chronic psychosocial stress protocol to model social gradients in aging and disease vulnerability. Low social rank, inferred based on a discretized aggression index, was found to negatively impact lifespan in our study population. However, social rank interacted with genetic background in that low-ranking C57BL/6J, high-ranking Sv129Ev, and middle-ranking CD1 mice had lower survival, respectively, implying a cost of maintaining a given social rank that varies across strains. Machine learning linear discriminant analysis identified baseline fat-free mass as the most important predictor of mouse genetic background and social rank in the present dataset. Finally, strain and social rank differences were significantly associated with epigenetic changes, most significantly in Sv129Ev mice and in high-ranking compared to lower ranking subjects. Overall, we identified genetic background and social rank as critical contextual modifiers of aging and lifespan in an ethologically relevant rodent model of social stress, thereby providing a preclinical experimental paradigm to study the impact of social determinants of health disparities and accelerated aging.
Keywords: aging; epigenetic; social determinants of health; social status; strain differences.
Conflict of interest statement
B.H.C. is a full-time employee of FOXO Technologies Inc., which seeks to commercialize epigenetic technologies in the life insurance industry. B.H.C. owns stock in Illumina Inc., the manufacturer of the DNA methylation arrays used in this study. B.H.C. is listed as a co-inventor in filed patents on commercial applications of epigenetic prediction models. The other authors declare no competing interest.
Figures



Similar articles
-
Social stress shortens lifespan in mice.Aging Cell. 2018 Aug;17(4):e12778. doi: 10.1111/acel.12778. Epub 2018 May 28. Aging Cell. 2018. PMID: 29806171 Free PMC article.
-
Psychosocial stress and epigenetic aging.Int Rev Neurobiol. 2020;150:107-128. doi: 10.1016/bs.irn.2019.10.020. Epub 2019 Nov 20. Int Rev Neurobiol. 2020. PMID: 32204828 Review.
-
A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated.Aging (Albany NY). 2016 Oct 2;8(10):2370-2391. doi: 10.18632/aging.101059. Aging (Albany NY). 2016. PMID: 27705904 Free PMC article.
-
Lifespan and lesions in genetically heterogeneous (four-way cross) mice: a new model for aging research.Vet Pathol. 1996 Nov;33(6):735-43. doi: 10.1177/030098589603300620. Vet Pathol. 1996. PMID: 8952040
-
Epigenetics as a key link between psychosocial stress and aging: concepts, evidence, mechanisms .Dialogues Clin Neurosci. 2019 Dec;21(4):389-396. doi: 10.31887/DCNS.2019.21.4/azannas. Dialogues Clin Neurosci. 2019. PMID: 31949406 Free PMC article. Review.
Cited by
-
Optimizing preclinical models of ageing for translation to clinical trials.Br J Clin Pharmacol. 2025 Jan;91(1):5-7. doi: 10.1111/bcp.15902. Epub 2023 Sep 21. Br J Clin Pharmacol. 2025. PMID: 37675638 Free PMC article. Review.
-
The fortunes and misfortunes of social life across the life course: A new era of research from field, laboratory and comparative studies.Neurosci Biobehav Rev. 2024 Jul;162:105655. doi: 10.1016/j.neubiorev.2024.105655. Epub 2024 Apr 5. Neurosci Biobehav Rev. 2024. PMID: 38583652 Free PMC article.
-
A Multiparadigm Approach to Characterize Dominance Behaviors in CD1 and C57BL6 Male Mice.eNeuro. 2024 Nov 22;11(11):ENEURO.0342-24.2024. doi: 10.1523/ENEURO.0342-24.2024. Print 2024 Nov. eNeuro. 2024. PMID: 39500574 Free PMC article.
-
Social disadvantage accelerates aging.Nat Med. 2025 May;31(5):1635-1643. doi: 10.1038/s41591-025-03563-4. Epub 2025 Mar 14. Nat Med. 2025. PMID: 40087516 Free PMC article.
-
To promote healthy aging, focus on the environment.Nat Aging. 2023 Nov;3(11):1334-1344. doi: 10.1038/s43587-023-00518-7. Epub 2023 Nov 9. Nat Aging. 2023. PMID: 37946045 Review.
References
-
- Olshansky S. J., From lifespan to healthspan. JAMA – J. Am. Med. Assoc. 320, 1323–1324 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

