Pleiotropic role of TRAF7 in skull-base meningiomas and congenital heart disease
- PMID: 37043537
- PMCID: PMC10120005
- DOI: 10.1073/pnas.2214997120
Pleiotropic role of TRAF7 in skull-base meningiomas and congenital heart disease
Abstract
While somatic variants of TRAF7 (Tumor necrosis factor receptor-associated factor 7) underlie anterior skull-base meningiomas, here we report the inherited mutations of TRAF7 that cause congenital heart defects. We show that TRAF7 mutants operate in a dominant manner, inhibiting protein function via heterodimerization with wild-type protein. Further, the shared genetics of the two disparate pathologies can be traced to the common origin of forebrain meninges and cardiac outflow tract from the TRAF7-expressing neural crest. Somatic and inherited mutations disrupt TRAF7-IFT57 interactions leading to cilia degradation. TRAF7-mutant meningioma primary cultures lack cilia, and TRAF7 knockdown causes cardiac, craniofacial, and ciliary defects in Xenopus and zebrafish, suggesting a mechanistic convergence for TRAF7-driven meningiomas and developmental heart defects.
Keywords: TRAF7; cilia; congenital heart defect; meningioma.
Conflict of interest statement
The authors declare no competing interest.
Figures



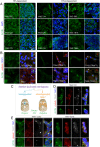
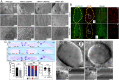

References
-
- Bouwmeester T., et al. , A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 6, 97–105 (2004). - PubMed
-
- Xu L.-G., Li L.-Y., Shu H.-B., TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J. Biol. Chem. 279, 17278–17282 (2004). - PubMed
-
- Yoshida H., Jono H., Kai H., Li J. D., The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J. Biol. Chem. 280, 41111–41121 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

