Targeting BRD3 eradicates nuclear TYRO3-induced colorectal cancer metastasis
- PMID: 37043564
- PMCID: PMC10096587
- DOI: 10.1126/sciadv.ade3422
Targeting BRD3 eradicates nuclear TYRO3-induced colorectal cancer metastasis
Erratum in
-
Erratum for the Research Article: "Targeting BRD3 eradicates nuclear TYRO3-induced colorectal cancer metastasis".Sci Adv. 2023 Nov 10;9(45):eadl3229. doi: 10.1126/sciadv.adl3229. Epub 2023 Nov 10. Sci Adv. 2023. PMID: 37948531 Free PMC article. No abstract available.
Abstract
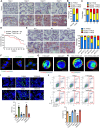
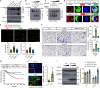
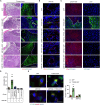
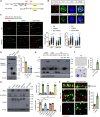
Metastasis is the main cause of death in many cancers including colorectal cancer (CRC); however, the underlying mechanisms responsible for metastatic progression remain largely unknown. We found that nuclear TYRO3 receptor tyrosine kinase is a strong predictor of poor overall survival in patients with CRC. The metastasis-promoting function of nuclear TYRO3 requires its kinase activity and matrix metalloproteinase-2 (MMP-2)-mediated cleavage but is independent of ligand binding. Using proteomic analysis, we identified bromodomain-containing protein 3 (BRD3), an acetyl-lysine reading epigenetic regulator, as one of nuclear TYRO3's substrates. Chromatin immunoprecipitation-sequencing data reveal that TYRO3-phosphorylated BRD3 regulates genes involved in anti-apoptosis and epithelial-mesenchymal transition. Inhibition of MMP-2 or BRD3 activity by selective inhibitors abrogates nuclear TYRO3-induced drug resistance and metastasis in organoid culture and in orthotopic mouse models. These data demonstrate that MMP-2/TYRO3/BRD3 axis promotes the metastasis of CRC, and blocking this signaling cascade is a promising approach to ameliorate CRC malignancy.
Figures
Similar articles
-
Tumor suppressive role of the epigenetic master regulator BRD3 in colorectal cancer.Cancer Sci. 2024 Jun;115(6):1866-1880. doi: 10.1111/cas.16129. Epub 2024 Mar 17. Cancer Sci. 2024. PMID: 38494600 Free PMC article.
-
TYRO3 promotes tumorigenesis and drug resistance in colorectal cancer by enhancing the epithelial-mesenchymal transition process.Aging (Albany NY). 2023 Apr 14;15(8):3035-3051. doi: 10.18632/aging.204656. Epub 2023 Apr 14. Aging (Albany NY). 2023. PMID: 37116196 Free PMC article.
-
MicroRNA-7 inhibits colorectal cancer cell proliferation, migration and invasion via TYRO3 and phosphoinositide 3-kinase/protein B kinase/mammalian target of rapamycin pathway suppression.Int J Mol Med. 2018 Nov;42(5):2503-2514. doi: 10.3892/ijmm.2018.3864. Epub 2018 Sep 7. Int J Mol Med. 2018. PMID: 30226533 Free PMC article.
-
FOXC2 promotes colorectal cancer metastasis by directly targeting MET.Oncogene. 2015 Aug 13;34(33):4379-90. doi: 10.1038/onc.2014.368. Epub 2014 Nov 10. Oncogene. 2015. PMID: 25381815
-
Histone Demethylase JMJD2D Interacts With β-Catenin to Induce Transcription and Activate Colorectal Cancer Cell Proliferation and Tumor Growth in Mice.Gastroenterology. 2019 Mar;156(4):1112-1126. doi: 10.1053/j.gastro.2018.11.036. Epub 2018 Nov 23. Gastroenterology. 2019. PMID: 30472235
Cited by
-
Epigenetic Modulators as Therapeutic Agents in Cancer.Int J Mol Sci. 2023 Oct 6;24(19):14964. doi: 10.3390/ijms241914964. Int J Mol Sci. 2023. PMID: 37834411 Free PMC article. Review.
-
CCZ1 Accelerates the Progression of Cervical Squamous Cell Carcinoma by Promoting MMP2/MMP17 Expression.Biomedicines. 2024 Jul 3;12(7):1468. doi: 10.3390/biomedicines12071468. Biomedicines. 2024. PMID: 39062041 Free PMC article.
-
A Review of the Bromodomain and Extraterminal Domain Epigenetic Reader Proteins: Function on Virus Infection and Cancer.Viruses. 2024 Jul 8;16(7):1096. doi: 10.3390/v16071096. Viruses. 2024. PMID: 39066258 Free PMC article. Review.
-
Epigenetic drivers of metalloproteinases and metastasis.Trends Cell Biol. 2025 Mar 14:S0962-8924(25)00044-3. doi: 10.1016/j.tcb.2025.02.010. Online ahead of print. Trends Cell Biol. 2025. PMID: 40089451 Review.
-
The application of organoids in colorectal diseases.Front Pharmacol. 2024 Jun 25;15:1412489. doi: 10.3389/fphar.2024.1412489. eCollection 2024. Front Pharmacol. 2024. PMID: 38983913 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

