Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis
- PMID: 37043568
- PMCID: PMC10096581
- DOI: 10.1126/sciadv.ade5041
Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis
Abstract
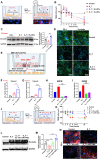
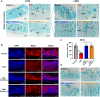
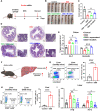
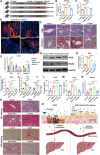
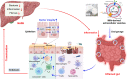
Milk-derived extracellular vesicles (mEVs) have been proposed as a potential nanomedicine for intestinal disorders; however, their impact on intestinal barrier integrity in gut inflammation and associated metabolic diseases has not been explored yet. Here, mEVs derived from bovine and human breast milk exert similar protective effects on epithelial tight junction functionality in vitro, survive harsh gastrointestinal conditions ex vivo, and reach the colon in vivo. Oral administration of mEVs restores gut barrier integrity at multiple levels, including mucus, epithelial, and immune barriers, and prevents endotoxin translocation into the liver in chemical-induced experimental colitis and diet-induced nonalcoholic steatohepatitis (NASH), thereby alleviating gut disorders, their associated liver inflammation, and NASH. Oral administration of mEVs has potential in the treatment of gut inflammation and gut-liver axis-associated metabolic diseases via protection of intestinal barrier integrity.
Figures





References
-
- J. R. Turner, Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009). - PubMed
-
- L. Miele, V. Valenza, G. La Torre, M. Montalto, G. Cammarota, R. Ricci, R. Masciana, A. Forgione, M. L. Gabrieli, G. Perotti, F. M. Vecchio, G. Rapaccini, G. Gasbarrini, C. P. Day, A. Grieco, Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009). - PubMed
-
- C. K. Heazlewood, M. C. Cook, R. Eri, G. R. Price, S. B. Tauro, D. Taupin, D. J. Thornton, C. W. Png, T. L. Crockford, R. J. Cornall, R. Adams, M. Kato, K. A. Nelms, N. A. Hong, T. H. Florin, C. C. Goodnow, M. A. McGuckin, Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLOS Med. 5, e54 (2008). - PMC - PubMed
-
- J. M. Allaire, S. M. Crowley, H. T. Law, S. Y. Chang, H. J. Ko, B. A. Vallance, The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 39, 677–696 (2018). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

