Tranexamic Acid to Prevent Obstetrical Hemorrhage after Cesarean Delivery
- PMID: 37043652
- PMCID: PMC10200294
- DOI: 10.1056/NEJMoa2207419
Tranexamic Acid to Prevent Obstetrical Hemorrhage after Cesarean Delivery
Abstract
Background: Prophylactic use of tranexamic acid at the time of cesarean delivery has been shown to decrease the calculated blood loss, but the effect on the need for blood transfusions is unclear.
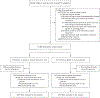
Methods: We randomly assigned patients undergoing cesarean delivery at 31 U.S. hospitals to receive either tranexamic acid or placebo after umbilical-cord clamping. The primary outcome was a composite of maternal death or blood transfusion by hospital discharge or 7 days post partum, whichever came first. Key secondary outcomes were estimated intraoperative blood loss of more than 1 liter (prespecified as a major secondary outcome), interventions for bleeding and related complications, the preoperative-to-postoperative change in the hemoglobin level, and postpartum infectious complications. Adverse events were assessed.
Results: A total of 11,000 participants underwent randomization (5529 to the tranexamic acid group and 5471 to the placebo group); scheduled cesarean delivery accounted for 50.1% and 49.2% of the deliveries in the respective groups. A primary-outcome event occurred in 201 of 5525 participants (3.6%) in the tranexamic acid group and in 233 of 5470 (4.3%) in the placebo group (adjusted relative risk, 0.89; 95.26% confidence interval [CI], 0.74 to 1.07; P = 0.19). Estimated intraoperative blood loss of more than 1 liter occurred in 7.3% of the participants in the tranexamic acid group and in 8.0% of those in the placebo group (relative risk, 0.91; 95% CI, 0.79 to 1.05). Interventions for bleeding complications occurred in 16.1% of the participants in the tranexamic acid group and in 18.0% of those in the placebo group (relative risk, 0.90; 95% CI, 0.82 to 0.97); the change in the hemoglobin level was -1.8 g per deciliter and -1.9 g per deciliter, respectively (mean difference, -0.1 g per deciliter; 95% CI, -0.2 to -0.1); and postpartum infectious complications occurred in 3.2% and 2.5% of the participants, respectively (relative risk, 1.28; 95% CI, 1.02 to 1.61). The frequencies of thromboembolic events and other adverse events were similar in the two groups.
Conclusions: Prophylactic use of tranexamic acid during cesarean delivery did not lead to a significantly lower risk of a composite outcome of maternal death or blood transfusion than placebo. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; ClinicalTrials.gov number, NCT03364491.).
Copyright © 2023 Massachusetts Medical Society.
Figures
References
-
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2(6): e323–e333. - PubMed
-
- Davis NL, Smoots AN, Goodman DA. Pregnancy-related deaths: data from 14 U.S. maternal mortality review committees, 2008–2017. Atlanta: Centers for Disease Control and Prevention, 2019. (https://www.cdc.gov/reproductivehealth/maternal-mortality/erase-mm/mmr-d...).
-
- CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010; 376: 23–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- UM1 TR004409/TR/NCATS NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
