In SARS-CoV-2 delta variants, Spike-P681R and D950N promote membrane fusion, Spike-P681R enhances spike cleavage, but neither substitution affects pathogenicity in hamsters
- PMID: 37043872
- PMCID: PMC10083686
- DOI: 10.1016/j.ebiom.2023.104561
In SARS-CoV-2 delta variants, Spike-P681R and D950N promote membrane fusion, Spike-P681R enhances spike cleavage, but neither substitution affects pathogenicity in hamsters
Abstract
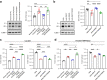
Background: The SARS-CoV-2 delta (B.1.617.2 lineage) variant was first identified at the end of 2020 and possessed two unique amino acid substitutions in its spike protein: S-P681R, at the S1/S2 cleavage site, and S-D950N, in the HR1 of the S2 subunit. However, the roles of these substitutions in virus phenotypes have not been fully characterized.
Methods: We used reverse genetics to generate Wuhan-D614G viruses with these substitutions and delta viruses lacking these substitutions and explored how these changes affected their viral characteristics in vitro and in vivo.
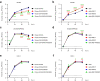
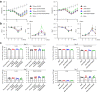
Findings: S-P681R enhanced spike cleavage and membrane fusion, whereas S-D950N slightly promoted membrane fusion. Although S-681R reduced the virus replicative ability especially in VeroE6 cells, neither substitution affected virus replication in Calu-3 cells and hamsters. The pathogenicity of all recombinant viruses tested in hamsters was slightly but not significantly affected.
Interpretation: Our observations suggest that the S-P681R and S-D950N substitutions alone do not increase virus pathogenicity, despite of their enhancement of spike cleavage or fusogenicity.
Funding: A full list of funding bodies that contributed to this study can be found under Acknowledgments.
Keywords: COVID-19; Hamster; Reverse genetics; SARS-CoV-2.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Y.K. has received collaborative research funds from the following companies. For antiviral studies; FUJIFILM Toyama Chemical Co. LTD (various viruses), Shionogi &; Co. LTD (influenza viruses). For vaccine and other studies; Daiichi Sankyo Pharmaceutical, Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Fuji Rebio, Tauns Laboratories, Inc., Matsubara Co. LTD. As the Flagship Center of the Japan Initiative for World-leading Vaccine Research and Development Centers, we plan to initiate collaborative research related to vaccine development with the following companies: Shionogi &Co. LTD, Daiichi Sankyo Pharmaceutical, KM Biologics, Cytiva, Sysmex Corporation, NEC.
Figures

References
-
- Singh J., Rahman S.A., Ehtesham N.Z., Hira S., Hasnain S.E. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021;27:1131–1133. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

