Multiple ulcers and perforation of small intestine with everolimus use in a patient with rectal neuroendocrine tumor: A case report
- PMID: 37043898
- PMCID: PMC10139960
- DOI: 10.1016/j.ijscr.2023.108094
Multiple ulcers and perforation of small intestine with everolimus use in a patient with rectal neuroendocrine tumor: A case report
Abstract
Introduction: Everolimus is an orally administered inhibitor of the mammalian target of rapamycin, which is a serine/threonine protein kinase. It is used for the treatment of pancreatic and gastrointestinal neuroendocrine tumors (NETs). Gastrointestinal perforations in patients being treated with everolimus is extremely rare, with only five reported cases.
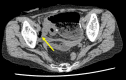
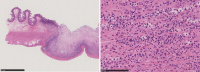
Case presentation: A 62-year-old woman, who had previously undergone surgery for rectal NET, presented to our hospital with fever and abdominal pain. Abdominal computed tomography revealed perforation of the lower gastrointestinal tract, and we performed emergency surgery. There were multiple ulcers 150 cm distal from the ligament of Treitz to the terminal ileum; an ulcer at the anastomosis of stoma closure, 35 cm from the terminal ileum, was transmural. We subsequently performed a partial intestinal resection.
Clinical discussion: The diagnosis of NETs is increasing worldwide, owing to recent improvements in diagnostic techniques. Although the use of everolimus has increased, gastrointestinal ulcer perforations caused by everolimus treatment have rarely been reported. The mechanism may be due to the inhibition of angiogenesis by mTOR inhibitors, as well as vascular endothelial growth factor inhibitors. In this case, It was considered that everolimus use most likely caused perforation.
Conclusion: It is necessary to recognize that drug-induced gastrointestinal ulcers and perforations may occur with the use of mTOR inhibitors, and careful follow-up should be performed during administration.
Keywords: Everolimus; Multiple ulcers; Perforation.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no conflict of interest.
Figures


Similar articles
-
An Unusual Case of Colon Perforation With Multiple Transmural Ulcers After Use of Polmacoxib and Everolimus in a Metastatic Breast Cancer Patient.Ann Coloproctol. 2021 Apr;37(2):120-124. doi: 10.3393/ac.2019.08.17. Epub 2020 Mar 16. Ann Coloproctol. 2021. PMID: 32178492 Free PMC article.
-
Aggressive neuroendocrine tumor of the ovary with multiple metastases treated with everolimus: A case report.Gynecol Oncol Rep. 2018 Jan 4;23:20-23. doi: 10.1016/j.gore.2018.01.002. eCollection 2018 Feb. Gynecol Oncol Rep. 2018. PMID: 29326972 Free PMC article.
-
Terminal Ileac Ulcers Mimicked Post-transplantation Lymphoproliferative Disorder in a Heart Recipient Treated With Everolimus: A Case Report.Transplant Proc. 2018 Dec;50(10):4053-4056. doi: 10.1016/j.transproceed.2018.08.025. Epub 2018 Sep 7. Transplant Proc. 2018. PMID: 30577313
-
Everolimus.Recent Results Cancer Res. 2018;211:101-123. doi: 10.1007/978-3-319-91442-8_8. Recent Results Cancer Res. 2018. PMID: 30069763 Review.
-
Everolimus and pancreatic neuroendocrine tumors (PNETs): Activity, resistance and how to overcome it.Int J Surg. 2015 Sep;21 Suppl 1:S89-94. doi: 10.1016/j.ijsu.2015.06.064. Epub 2015 Jun 27. Int J Surg. 2015. PMID: 26123382 Review.
References
-
- Okuyama Y., Honda A., Ri T., Nakatugawa Y., Yamada S., Suzuki T. Everolimus induced colitis and ulcers, report of a case. Stomach Intestine. 2020;7(55):946–950.
-
- Paplomata E., Zelnak A., O’Regan R. Everolimus: side effect profile and management of toxicities in breast cancer. Breast Cancer Res. Treat. 2013;140(3):453–462. - PubMed
-
- Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical Case Report (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. - PubMed
-
- Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., SCARE Group The PROCESS 2018 statement: updating consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int. J. Surg. 2018;60:279–282. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

