Scalable generation of sensory neurons from human pluripotent stem cells
- PMID: 37044067
- PMCID: PMC10147831
- DOI: 10.1016/j.stemcr.2023.03.006
Scalable generation of sensory neurons from human pluripotent stem cells
Abstract
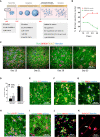
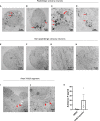
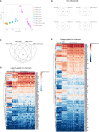
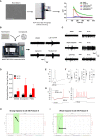
Development of new non-addictive analgesics requires advanced strategies to differentiate human pluripotent stem cells (hPSCs) into relevant cell types. Following principles of developmental biology and translational applicability, here we developed an efficient stepwise differentiation method for peptidergic and non-peptidergic nociceptors. By modulating specific cell signaling pathways, hPSCs were first converted into SOX10+ neural crest, followed by differentiation into sensory neurons. Detailed characterization, including ultrastructural analysis, confirmed that the hPSC-derived nociceptors displayed cellular and molecular features comparable to native dorsal root ganglion (DRG) neurons, and expressed high-threshold primary sensory neuron markers, transcription factors, neuropeptides, and over 150 ion channels and receptors relevant for pain research and axonal growth/regeneration studies (e.g., TRPV1, NAV1.7, NAV1.8, TAC1, CALCA, GAP43, DPYSL2, NMNAT2). Moreover, after confirming robust functional activities and differential response to noxious stimuli and specific drugs, a robotic cell culture system was employed to produce large quantities of human sensory neurons, which can be used to develop nociceptor-selective analgesics.
Keywords: CEPT cocktail; analgesics; cell differentiation; drug testing; iPS cells; neural crest; nociceptor; opioid crisis; pain; sensory neuron.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interests T.D., A.S., and I.S. are co-inventors on a US Department of Health and Human Services patent covering the nociceptor differentiation method and its utilization.
Figures



References
-
- Black B.J., Atmaramani R., Kumaraju R., Plagens S., Romero-Ortega M., Dussor G., Price T.J., Campbell Z.T., Pancrazio J.J. Adult mouse sensory neurons on microelectrode arrays exhibit increased spontaneous and stimulus-evoked activity in the presence of interleukin-6. J. Neurophysiol. 2018;120:1374–1385. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

