Changes in Reported Dietary Supplement Use in Cognitively Normal National Alzheimer's Coordinating Center Participants Aged 55 and Older From 2015 to 2019
- PMID: 37044210
- PMCID: PMC10447883
- DOI: 10.1016/j.tjnut.2023.04.004
Changes in Reported Dietary Supplement Use in Cognitively Normal National Alzheimer's Coordinating Center Participants Aged 55 and Older From 2015 to 2019
Abstract
Background: Although reported dietary supplement use is common in older adults, evaluations of dietary supplement use over the past 10 y are lacking.
Objective: This analysis determined changes in reported dietary supplement use in cognitively normal older adults (aged ≥ 55 y) using the National Alzheimer's Coordinating Center data from 2015 to 2019 using a serial cross-sectional study design.
Methods: The first available visit for cognitively normal participants aged ≥ 55 y from 2015 to 2019 with a complete medication form was used, resulting in 9357 participants. Associations between visit year categories and reported use of dietary supplement categories/individual supplements were tested using categorical statistics. To determine whether the probabilities of reported supplement use changed in 2019 compared with those of 2015, z-scores and two-sided P values were used. Weighted analyses were used to confirm analytical findings.
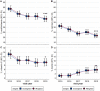
Results: When comparing 2015 and 2019, the reported use of any dietary supplement decreased from 77.7% in 2015 to 71.0% in 2019 (P < 0.0001); any vitamin from 72.5% to 65.5% (P < 0.0001); any mineral from 39.2% to 30.4% (P < 0.0001); "other" nonvitamin/nonmineral supplements from 34.4% to 26.9% (P < 0.0001), calcium from 31.2% to 21.7% (P < 0.0001), multivitamins from 48.4% to 38.4% (P < 0.0001), potassium from 5.6% to 3.5% (P = 0.001), vitamin C from 13.0% to 9.2% (P = 0.0002), chondroitin from 6.0% to 4.1% (P = 0.006), glucosamine from 11.1% to 6.5% (P < 0.0001), and all omega fatty acids from 25.2% to 17.0% (P < 0.0001). Reported use increased for vitamin B7/biotin from 3.1% in 2015 to 5.8% in 2019 (P = 0.0003), melatonin from 3.1% to 5.8% (P = 0.0002), and turmeric from 1.2% to 4.7% (P < 0.0001).
Conclusion: Although the reported use of many major dietary supplement categories and individual supplements significantly decreased in older adults from 2015 to 2019, biotin, turmeric, and melatonin significantly increased. Because biotin may interfere with some laboratory tests, this may have important public health implications.
Keywords: NACC; dietary supplement; dietary supplements; herbal; mineral; older adults; supplement; vitamins.
Copyright © 2023 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Congress U. Public Law; 1994. Dietary supplement health and education act of 1994; pp. 4325–4335.https://ods.od.nih.gov/About/DSHEA_Wording.aspx
-
- Timbo B.B., Chirtel S.J., Ihrie J., Oladipo T., Velez-Suarez L., Brewer V., et al. Dietary supplement adverse event report data from the FDA Center for food safety and applied nutrition adverse event reporting system (CAERS), 2004-2013. Ann. Pharmacother. 2018;52(5):431–438. doi: 10.1177/1060028017744316. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 AG068053/AG/NIA NIH HHS/United States
- P30 AG066515/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- P30 AG072973/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- P20 AG068077/AG/NIA NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
- P20 AG068082/AG/NIA NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- P30 AG066518/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- P30 AG072978/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- R01 AG079280/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- P20 AG068024/AG/NIA NIH HHS/United States
- P30 AG072958/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG066506/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- P30 AG072931/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

