Characterization of the endotheliopathy, innate-immune activation and hemostatic imbalance underlying CAR-T cell toxicities: laboratory tools for an early and differential diagnosis
- PMID: 37045474
- PMCID: PMC10106034
- DOI: 10.1136/jitc-2022-006365
Characterization of the endotheliopathy, innate-immune activation and hemostatic imbalance underlying CAR-T cell toxicities: laboratory tools for an early and differential diagnosis
Abstract
Background: Chimeric antigen receptor (CAR)-T cell-based immunotherapy constitutes a revolutionary advance for treatment of relapsed/refractory hematological malignancies. Nevertheless, cytokine release and immune effector cell-associated neurotoxicity syndromes are life-threatening toxicities in which the endothelium could be a pathophysiological substrate. Furthermore, differential diagnosis from sepsis, highly incident in these patients, is challenging. Suitable laboratory tools could be determinant for their appropriate management.
Methods: Sixty-two patients treated with CAR-T cell immunotherapy for hematological malignancies (n=46 with CD19-positive diseases, n=16 with multiple myeloma) were included. Plasma samples were obtained: before CAR-T cell infusion (baseline); after 24-48 hours; at suspicion of any toxicity onset and 24-48 hours after immunomodulatory treatment. Biomarkers of endothelial dysfunction (soluble vascular cell adhesion molecule 1 (sVCAM-1), soluble TNF receptor 1 (sTNFRI), thrombomodulin (TM), soluble suppression of tumorigenesis-2 factor (ST2), angiopoietin-2 (Ang-2)), innate immunity activation (neutrophil extracellular traps (NETs), soluble C5b-9 (sC5b-9)) and hemostasis/fibrinolysis (von Willebrand Factor antigen (VWF:Ag), ADAMTS-13 (A13), α2-antiplasmin (α2-AP), plasminogen activator inhibitor-1 antigen (PAI-1 Ag)) were measured and compared with those in cohorts of patients with sepsis and healthy donors.
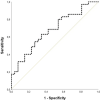
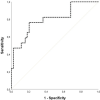
Results: Patients who developed CAR-T cell toxicities presented increased levels of sVCAM-1, sTNFRI and ST2 at the clinical onset versus postinfusion values. Twenty-four hours after infusion, ST2 levels were good predictors of any CAR-T cell toxicity, and combination of ST2, Ang-2 and NETs differentiated patients requiring intensive care unit admission from those with milder clinical presentations. Association of Ang-2, NETs, sC5b-9, VWF:Ag and PAI-1 Ag showed excellent discrimination between severe CAR-T cell toxicities and sepsis.
Conclusions: This study provides relevant contributions to the current knowledge of the CAR-T cell toxicities pathophysiology. Markers of endotheliopathy, innate immunity activation and hemostatic imbalance appear as potential laboratory tools for their prediction, severity and differential diagnosis.
Keywords: antineoplastic protocols; cytotoxicity, immunologic; hematologic neoplasms; immunity, cellular; immunotherapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MD-R and EC have been granted by and received honoraria from Jazz Pharmaceuticals. SF and PC have collaborated with Jansen, Gilead, Kite, MSD, Alexion and Pfizer, outside of the submitted work. MP received speaker’s fee from Jazz Pharmaceuticals. The rest of authors have no competing interests to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
