Use of norepinephrine in preterm neonates with dopamine-resistant shock: a retrospective single-centre cross-sectional study
- PMID: 37045542
- PMCID: PMC10106054
- DOI: 10.1136/bmjpo-2022-001804
Use of norepinephrine in preterm neonates with dopamine-resistant shock: a retrospective single-centre cross-sectional study
Abstract
Background: Norepinephrine (NE) is recommended for children and full-term neonates (born at >37 gestational weeks) with septic shock. Meanwhile, data on the effectiveness of NE in preterm neonates are still limited. This study aimed to evaluate the clinical efficacy of NE in preterm neonates with dopamine-resistant shock compared with that in full-term neonates.
Methods: This was a single-centre, retrospective (January 2010-December 2020) cohort study of neonates with persistent shock despite adequate fluid resuscitation and dopamine or dobutamine administration at ≥10 μg/kg/min. Medical records of neonates treated with NE were retrospectively reviewed to collect respiratory and haemodynamic parameters and results of arterial blood gas (ABG) tests before and 8 hours after NE infusion. The effectiveness of NE was assessed using changes in clinical parameters and multiple regression models for mortality among subgroups of preterm and full-term neonates.
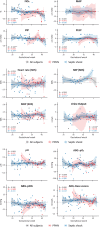
Results: Ninety-two neonates (76% preterm) who received NE infusion were included in the study. NE infusion was started after a median of 7 hours (IQR 2-19 hours) after shock onset. Among the preterm neonates, the maximum dose of NE infusion was 0.5 (IQR 0.3-1.0) µg/kg/min with a median duration of 45 (IQR 24.0-84.5) hours. Haemodynamic dysfunction was ameliorated with increased blood pressure, decreased heart rate and improved ABG results. Preterm neonates with septic shock tended to have a reduced response to NE; however, preterm neonates with persistent pulmonary hypertension of the newborn tended to have a better response. Thirty-four (37%) neonates died in our cohort. The timing, dose and duration of NE use were not associated with neonatal mortality.
Conclusions: Although using NE effectively improves clinical parameters in preterm neonates with dopamine-resistant shock, our study is underpowered to identify the association between NE infusion and mortality in preterm neonates with dopamine-resistant shock.
Keywords: Neonatology; Resuscitation.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
The effect of norepinephrine on clinical and hemodynamic parameters in neonates with shock: a retrospective cohort study.Eur J Pediatr. 2022 Jun;181(6):2379-2387. doi: 10.1007/s00431-022-04437-4. Epub 2022 Mar 11. Eur J Pediatr. 2022. PMID: 35277734
-
Dopamine or norepinephrine for sepsis-related hypotension in preterm infants: a retrospective cohort study.Eur J Pediatr. 2023 Mar;182(3):1029-1038. doi: 10.1007/s00431-022-04758-4. Epub 2022 Dec 22. Eur J Pediatr. 2023. PMID: 36544000
-
Norepinephrine infusion improves haemodynamics in the preterm infants during septic shock.Acta Paediatr. 2018 Mar;107(3):408-413. doi: 10.1111/apa.14112. Epub 2017 Oct 26. Acta Paediatr. 2018. PMID: 28992392
-
Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome.Pediatrics. 2003 Oct;112(4):793-9. doi: 10.1542/peds.112.4.793. Pediatrics. 2003. PMID: 14523168 Review.
-
Dopamine and dobutamine in pediatric therapy.Pharmacotherapy. 1989;9(5):303-14. doi: 10.1002/j.1875-9114.1989.tb04142.x. Pharmacotherapy. 1989. PMID: 2682552 Review.
Cited by
-
Development of a core outcome set for neonatal septic shock management: a study protocol.Trials. 2024 Oct 29;25(1):729. doi: 10.1186/s13063-024-08422-0. Trials. 2024. PMID: 39473018 Free PMC article.
-
Dose-related systemic and cerebral hemodynamic effects of norepinephrine in newborn piglets with hypoxia-reoxygenation.Pediatr Res. 2025 Apr 3. doi: 10.1038/s41390-025-04010-3. Online ahead of print. Pediatr Res. 2025. PMID: 40181145
-
Cardiorespiratory interactions during the transitional period in extremely preterm infants: a narrative review.Pediatr Res. 2025 Feb;97(3):871-879. doi: 10.1038/s41390-024-03451-6. Epub 2024 Aug 23. Pediatr Res. 2025. PMID: 39179873 Review.
-
An Update on Pharmacologic Management of Neonatal Hypotension: When, Why, and Which Medication.Children (Basel). 2024 Apr 19;11(4):490. doi: 10.3390/children11040490. Children (Basel). 2024. PMID: 38671707 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
