Targeting neuronal lysosomal dysfunction caused by β-glucocerebrosidase deficiency with an enzyme-based brain shuttle construct
- PMID: 37045813
- PMCID: PMC10097658
- DOI: 10.1038/s41467-023-37632-4
Targeting neuronal lysosomal dysfunction caused by β-glucocerebrosidase deficiency with an enzyme-based brain shuttle construct
Abstract
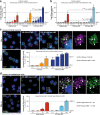
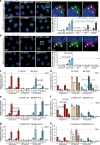
Mutations in glucocerebrosidase cause the lysosomal storage disorder Gaucher's disease and are the most common risk factor for Parkinson's disease. Therapies to restore the enzyme's function in the brain hold great promise for treating the neurological implications. Thus, we developed blood-brain barrier penetrant therapeutic molecules by fusing transferrin receptor-binding moieties to β-glucocerebrosidase (referred to as GCase-BS). We demonstrate that these fusion proteins show significantly increased uptake and lysosomal efficiency compared to the enzyme alone. In a cellular disease model, GCase-BS rapidly rescues the lysosomal proteome and lipid accumulations beyond known substrates. In a mouse disease model, intravenous injection of GCase-BS leads to a sustained reduction of glucosylsphingosine and can lower neurofilament-light chain plasma levels. Collectively, these findings demonstrate the potential of GCase-BS for treating GBA1-associated lysosomal dysfunction, provide insight into candidate biomarkers, and may ultimately open a promising treatment paradigm for lysosomal storage diseases extending beyond the central nervous system.
© 2023. The Author(s).
Conflict of interest statement
The following authors have been employed at or collaborated with F. Hoffmann-La Roche AG while working on this project: Alexandra Gehrlein, Vinod Udayar, Nadia Anastasi, Martino Luca Morella, Iris Ruf, Doris Brugger, Sophia von der Mark, Ralf Thoma, Arne Rufer, Dominik Heer, Nina Pfahler, Anton Jochner, Jens Niewoehner, Luise Wolf, Matthias Fueth, Martin Ebeling, Roberto Villaseñor, Yanping Zhu, Matthew C. Deen, Xiaoyang Shan, Zahra Ehsaei, Verdon Taylor, Ellen Sidransky, David J. Vocadlo, Per-Ola Freskgård, Ravi Jagasia. All authors declare no further competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

