PKD phosphorylation and COP9/Signalosome modulate intracellular Spry2 protein stability
- PMID: 37045830
- PMCID: PMC10097667
- DOI: 10.1038/s41389-023-00465-3
PKD phosphorylation and COP9/Signalosome modulate intracellular Spry2 protein stability
Abstract
Spry2 is a molecular modulator of tyrosine kinase receptor signaling pathways that has cancer-type-specific effects. Mammalian Spry2 protein undergoes tyrosine and serine phosphorylation in response to growth factor stimulation. Spry2 expression is distinctly altered in various cancer types. Inhibition of the proteasome functionality results in reduced intracellular Spry2 degradation. Using in vitro and in vivo assays, we show that protein kinase D (PKD) phosphorylates Spry2 at serine 112 and interacts in vivo with the C-terminal half of this protein. Importantly, missense mutation of Ser112 decreases the rate of Spry2 intracellular protein degradation. Either knocking down the expression of all three mammalian PKD isoforms or blocking their kinase activity with a specific inhibitor contributes to the stabilization of Spry2 wild-type protein. Downregulation of CSN3, a component of the COP9/Signalosome that binds PKD, significantly increases the half-life of Spry2 wild-type protein but does not affect the stability of a Spry2 after mutating Ser112 to the non-phosphorylatable residue alanine. Our data demonstrate that both PKD and the COP9/Signalosome play a significant role in control of Spry2 intracellular stability and support the consideration of the PKD/COP9 complex as a potential therapeutic target in tumors where Spry2 expression is reduced.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

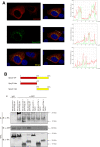


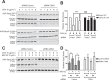
References
Grants and funding
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- CGB14143035THOM/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- PI20CIII/00029/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI20CIII/00029/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI20CIII/00029/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI19/00934/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- CB16/12/00352/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI20CIII/00019/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI20CIII/00019/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- ISCIII-CIBERNED/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI19/00934/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- CB16/12/00352/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI20CIII/00029/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- SAF2016-78852-R/Ministerio de Economía y Competitividad (Ministry of Economy and Competitiveness)
- SAF2016-78852-R/Ministerio de Economía y Competitividad (Ministry of Economy and Competitiveness)
- SAF2016-78852-R/Ministerio de Economía y Competitividad (Ministry of Economy and Competitiveness)
- SAF2016-78852-R/Ministerio de Economía y Competitividad (Ministry of Economy and Competitiveness)
- SA264P18-UIC 076/Consejería de Educación, Junta de Castilla y León (Consejería de Educación de la Junta de Castilla y León)
- SA264P18-UIC 076/Consejería de Educación, Junta de Castilla y León (Consejería de Educación de la Junta de Castilla y León)
- CIVP19A5942/Fundación Ramón Areces (Ramón Areces Foundation)
- CIVP19A5942/Fundación Ramón Areces (Ramón Areces Foundation)
- PID2020-115218RB-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
LinkOut - more resources
Full Text Sources
Miscellaneous

