The RNA-binding protein landscapes differ between mammalian organs and cultured cells
- PMID: 37045843
- PMCID: PMC10097726
- DOI: 10.1038/s41467-023-37494-w
The RNA-binding protein landscapes differ between mammalian organs and cultured cells
Abstract
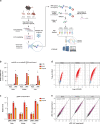
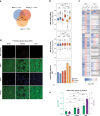
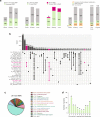
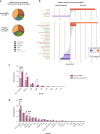
System-wide approaches have unveiled an unexpected breadth of the RNA-bound proteomes of cultured cells. Corresponding information regarding RNA-binding proteins (RBPs) of mammalian organs is still missing, largely due to technical challenges. Here, we describe ex vivo enhanced RNA interactome capture (eRIC) to characterize the RNA-bound proteomes of three different mouse organs. The resulting organ atlases encompass more than 1300 RBPs active in brain, kidney or liver. Nearly a quarter (291) of these had formerly not been identified in cultured cells, with more than 100 being metabolic enzymes. Remarkably, RBP activity differs between organs independent of RBP abundance, suggesting organ-specific levels of control. Similarly, we identify systematic differences in RNA binding between animal organs and cultured cells. The pervasive RNA binding of enzymes of intermediary metabolism in organs points to tightly knit connections between gene expression and metabolism, and displays a particular enrichment for enzymes that use nucleotide cofactors. We describe a generically applicable refinement of the eRIC technology and provide an instructive resource of RBPs active in intact mammalian organs, including the brain.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Human protein-RNA interaction network is highly stable across mammals.BMC Genomics. 2019 Dec 30;20(Suppl 12):1004. doi: 10.1186/s12864-019-6330-9. BMC Genomics. 2019. PMID: 31888461 Free PMC article.
-
Discovering the RNA-Binding Proteome of Plant Leaves with an Improved RNA Interactome Capture Method.Biomolecules. 2020 Apr 24;10(4):661. doi: 10.3390/biom10040661. Biomolecules. 2020. PMID: 32344669 Free PMC article.
-
The Cardiomyocyte RNA-Binding Proteome: Links to Intermediary Metabolism and Heart Disease.Cell Rep. 2016 Aug 2;16(5):1456-1469. doi: 10.1016/j.celrep.2016.06.084. Epub 2016 Jul 21. Cell Rep. 2016. PMID: 27452465 Free PMC article.
-
Ribonomic approaches to study the RNA-binding proteome.FEBS Lett. 2014 Oct 16;588(20):3649-64. doi: 10.1016/j.febslet.2014.07.039. Epub 2014 Aug 19. FEBS Lett. 2014. PMID: 25150170 Review.
-
The expanding world of metabolic enzymes moonlighting as RNA binding proteins.Biochem Soc Trans. 2021 Jun 30;49(3):1099-1108. doi: 10.1042/BST20200664. Biochem Soc Trans. 2021. PMID: 34110361 Review.
Cited by
-
New therapeutic approaches for fibrosis: harnessing translational regulation.Trends Mol Med. 2025 Jul;31(7):640-651. doi: 10.1016/j.molmed.2024.11.012. Epub 2024 Dec 16. Trends Mol Med. 2025. PMID: 39690057 Free PMC article. Review.
-
A widely applicable and cost-effective method for specific RNA-protein complex isolation.Sci Rep. 2023 Apr 27;13(1):6898. doi: 10.1038/s41598-023-34157-0. Sci Rep. 2023. PMID: 37106019 Free PMC article.
-
The impact of IDR phosphorylation on the RNA binding profiles of proteins.Trends Genet. 2024 Jul;40(7):580-586. doi: 10.1016/j.tig.2024.04.004. Epub 2024 May 4. Trends Genet. 2024. PMID: 38705823 Free PMC article. Review.
-
Host RNA-Binding Proteins as Regulators of HIV-1 Replication.Viruses. 2024 Dec 31;17(1):43. doi: 10.3390/v17010043. Viruses. 2024. PMID: 39861832 Free PMC article. Review.
-
Role of the RNA-binding protein family in gynecologic cancers.Am J Cancer Res. 2023 Aug 15;13(8):3799-3821. eCollection 2023. Am J Cancer Res. 2023. PMID: 37693158 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

