A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury
- PMID: 37045845
- PMCID: PMC10091329
- DOI: 10.1038/s41467-023-37845-7
A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury
Abstract
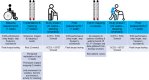
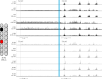
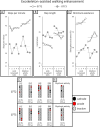
Two persons with chronic motor complete spinal cord injury (SCI) were implanted with percutaneous spinal cord epidural stimulation (SCES) leads to enable motor control below the injury level (NCT04782947). Through a period of temporary followed by permanent SCES implantation, spinal mapping was conducted primarily to optimize configurations enabling volitional control of movement and training of standing and stepping as a secondary outcome. In both participants, SCES enabled voluntary increased muscle activation and movement below the injury and decreased assistance during exoskeleton-assisted walking. After permanent implantation, both participants voluntarily modulated induced torques but not always in the intended directions. In one participant, percutaneous SCES enabled motor control below the injury one-day following temporary implantation as confirmed by electromyography. The same participant achieved independent standing with minimal upper extremity self-balance assistance, independent stepping in parallel bars and overground ambulation with a walker. SCES via percutaneous leads holds promise for enhancing rehabilitation and enabling motor functions for people with SCI.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury.J Clin Med. 2024 Nov 13;13(22):6826. doi: 10.3390/jcm13226826. J Clin Med. 2024. PMID: 39597970 Free PMC article.
-
Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic.Sci Rep. 2017 Oct 26;7(1):13476. doi: 10.1038/s41598-017-14003-w. Sci Rep. 2017. PMID: 29074997 Free PMC article.
-
Interleaved configurations of percutaneous epidural stimulation enhanced overground stepping in a person with chronic paraplegia.Front Neurosci. 2023 Dec 7;17:1284581. doi: 10.3389/fnins.2023.1284581. eCollection 2023. Front Neurosci. 2023. PMID: 38144208 Free PMC article.
-
Emergence of Epidural Electrical Stimulation to Facilitate Sensorimotor Network Functionality After Spinal Cord Injury.Neuromodulation. 2019 Apr;22(3):244-252. doi: 10.1111/ner.12938. Epub 2019 Mar 6. Neuromodulation. 2019. PMID: 30840354 Review.
-
Spinal Cord Stimulation and Augmentative Control Strategies for Leg Movement after Spinal Paralysis in Humans.CNS Neurosci Ther. 2016 Apr;22(4):262-70. doi: 10.1111/cns.12530. Epub 2016 Feb 18. CNS Neurosci Ther. 2016. PMID: 26890324 Free PMC article. Review.
Cited by
-
Epidural Stimulation and Resistance Training (REST-SCI) for Overground Locomotion After Spinal Cord Injury: Randomized Clinical Trial Protocol.J Clin Med. 2025 Mar 8;14(6):1829. doi: 10.3390/jcm14061829. J Clin Med. 2025. PMID: 40142643 Free PMC article.
-
Deep Brain Stimulation and Brain-Spine Interface for Functional Restoration in Spinal Cord Injury.Biomedicines. 2025 Mar 5;13(3):631. doi: 10.3390/biomedicines13030631. Biomedicines. 2025. PMID: 40149607 Free PMC article. Review.
-
Effects of exoskeleton-assisted walking on bowel function in motor-complete spinal cord injury patients: involvement of the brain-gut axis, a pilot study.Front Neurosci. 2024 Jun 17;18:1395671. doi: 10.3389/fnins.2024.1395671. eCollection 2024. Front Neurosci. 2024. PMID: 38952922 Free PMC article.
-
Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury.J Clin Med. 2024 Feb 27;13(5):1344. doi: 10.3390/jcm13051344. J Clin Med. 2024. PMID: 38592158 Free PMC article.
-
Consumer views of functional electrical stimulation and robotic exoskeleton in SCI rehabilitation: A mini review.Artif Organs. 2025 May;49(5):729-748. doi: 10.1111/aor.14925. Epub 2024 Dec 23. Artif Organs. 2025. PMID: 39711332 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

