OpcA and PorB are novel bactericidal antigens of the 4CMenB vaccine in mice and humans
- PMID: 37045859
- PMCID: PMC10097807
- DOI: 10.1038/s41541-023-00651-9
OpcA and PorB are novel bactericidal antigens of the 4CMenB vaccine in mice and humans
Abstract
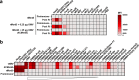
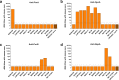
The ability of Neisseria meningitidis Outer Membrane Vesicles (OMV) to induce protective responses in humans is well established and mainly attributed to Porin A (PorA). However, the contribution of additional protein antigens to protection remains to be elucidated. In this study we dissected the immunogenicity of antigens originating from the OMV component of the 4CMenB vaccine in mice and humans. We collected functional data on a panel of strains for which bactericidal responses to 4CMenB in infants was attributable to the OMV component and evaluated the role of 30 OMV-specific protein antigens in cross-coverage. By using tailor-made protein microarrays, the immunosignature of OMV antigens was determined. Three of these proteins, OpcA, NspA, and PorB, triggered mouse antibodies that were bactericidal against several N. meningitidis strains. Finally, by genetic deletion and/or serum depletion studies, we demonstrated the ability of OpcA and PorB to induce functional immune responses in infant sera after vaccination. In conclusion, while confirming the role of PorA in eliciting protective immunity, we identified two OMV antigens playing a key role in protection of infants vaccinated with the 4CMenB vaccine against different N. meningitidis serogroup B strains.
© 2023. The Author(s).
Conflict of interest statement
V.V. was a PhD student at the University of Bologna Alma Mater Studiorum and participated in a post graduate studentship program at GSK, Siena, Italy at the time of the study and she is now an employee of the GSK group of companies. A.F., S.T., S.M., E.L., M.B., D.M., I.D., A.B., and E.B. are employees of the GSK group of companies. M.P. was an employee of the GSK group of companies at the time of the study and now is at Imperial College, South Kensington Campus, London, UK. M.M.G. was an employee of the GSK group of companies at the time of the study and now is on her retirement. J.P.D. is an employee of University of Manchester, Faculty of Biology, Medicine and Health. I.D. reports ownership of GSK shares.
Figures



References
-
- Sierra GV, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

