An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters
- PMID: 37045873
- PMCID: PMC10092940
- DOI: 10.1038/s41467-023-37697-1
An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters
Abstract
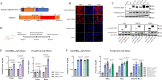
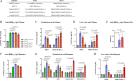
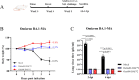
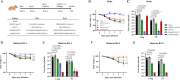
Current available vaccines for COVID-19 are effective in reducing severe diseases and deaths caused by SARS-CoV-2 infection but less optimal in preventing infection. Next-generation vaccines which are able to induce mucosal immunity in the upper respiratory to prevent or reduce infections caused by highly transmissible variants of SARS-CoV-2 are urgently needed. We have developed an intranasal vaccine candidate based on a live attenuated influenza virus (LAIV) with a deleted NS1 gene that encodes cell surface expression of the receptor-binding-domain (RBD) of the SARS-CoV-2 spike protein, designated DelNS1-RBD4N-DAF. Immune responses and protection against virus challenge following intranasal administration of DelNS1-RBD4N-DAF vaccines were analyzed in mice and compared with intramuscular injection of the BioNTech BNT162b2 mRNA vaccine in hamsters. DelNS1-RBD4N-DAF LAIVs induced high levels of neutralizing antibodies against various SARS-CoV-2 variants in mice and hamsters and stimulated robust T cell responses in mice. Notably, vaccination with DelNS1-RBD4N-DAF LAIVs, but not BNT162b2 mRNA, prevented replication of SARS-CoV-2 variants, including Delta and Omicron BA.2, in the respiratory tissues of animals. The DelNS1-RBD4N-DAF LAIV system warrants further evaluation in humans for the control of SARS-CoV-2 transmission and, more significantly, for creating dual function vaccines against both influenza and COVID-19 for use in annual vaccination strategies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the University of Hong Kong has filed patents on work related to the generation and application of DelNS1 live attenuated influenza vaccines and the associated platform, with H.C., P.W., and K-Y.Y. included as co-inventors. There is no restriction on the publication of data. The other authors declare that they have no competing interests.
Figures

Similar articles
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
-
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge.PLoS Pathog. 2025 Apr 21;21(4):e1012585. doi: 10.1371/journal.ppat.1012585. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40258004 Free PMC article.
-
Measles Virus-Based Vaccine Expressing Membrane-Anchored Spike of SARS-CoV-2 Inducing Efficacious Systemic and Mucosal Humoral Immunity in Hamsters.Viruses. 2024 Apr 3;16(4):559. doi: 10.3390/v16040559. Viruses. 2024. PMID: 38675901 Free PMC article.
-
Clinical development of variant-adapted BNT162b2 COVID-19 vaccines: the early Omicron era.Expert Rev Vaccines. 2023 Jan-Dec;22(1):650-661. doi: 10.1080/14760584.2023.2232851. Expert Rev Vaccines. 2023. PMID: 37417000 Review.
-
Intranasal COVID-19 vaccines: From bench to bed.EBioMedicine. 2022 Feb;76:103841. doi: 10.1016/j.ebiom.2022.103841. Epub 2022 Jan 24. EBioMedicine. 2022. PMID: 35085851 Free PMC article. Review.
Cited by
-
Intranasal B5 promotes mucosal defence against Actinobacillus pleuropneumoniae via ameliorating early immunosuppression.Virulence. 2024 Dec;15(1):2316459. doi: 10.1080/21505594.2024.2316459. Epub 2024 Feb 20. Virulence. 2024. PMID: 38378464 Free PMC article.
-
Bioprocessing and the Production of Antiviral Biologics in the Prevention and Treatment of Viral Infectious Disease.Vaccines (Basel). 2023 May 17;11(5):992. doi: 10.3390/vaccines11050992. Vaccines (Basel). 2023. PMID: 37243096 Free PMC article. Review.
-
Novel vaccine strategies to induce respiratory mucosal immunity: advances and implications.MedComm (2020). 2025 Jan 16;6(2):e70056. doi: 10.1002/mco2.70056. eCollection 2025 Feb. MedComm (2020). 2025. PMID: 39830020 Free PMC article. Review.
-
SARS-CoV-2 infection primes cross-protective respiratory IgA in a MyD88- and MAVS-dependent manner.NPJ Vaccines. 2025 Feb 27;10(1):40. doi: 10.1038/s41541-025-01095-z. NPJ Vaccines. 2025. PMID: 40016252 Free PMC article.
-
Progress in combination vaccines and the co-administration of influenza virus and SARS-CoV-2 vaccines.Front Immunol. 2025 Jun 25;16:1578733. doi: 10.3389/fimmu.2025.1578733. eCollection 2025. Front Immunol. 2025. PMID: 40636108 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

