Tumour budding-based grading as independent prognostic biomarker in HPV-positive and HPV-negative head and neck cancer
- PMID: 37045906
- PMCID: PMC10241901
- DOI: 10.1038/s41416-023-02240-y
Tumour budding-based grading as independent prognostic biomarker in HPV-positive and HPV-negative head and neck cancer
Erratum in
-
Correction: Tumour budding-based grading as independent prognostic biomarker in HPV-positive and HPV-negative head and neck cancer.Br J Cancer. 2024 Feb;130(3):511. doi: 10.1038/s41416-023-02521-6. Br J Cancer. 2024. PMID: 38191610 Free PMC article. No abstract available.
Abstract
Background: The prognostic significance of tumour budding (TB) and minimal cell nest size (MCNS) was shown in human papillomavirus (HPV)-negative head and neck squamous cell carcinomas (HNSCC). However, the optimisation of cutpoints, the prognostic impact in HPV-positive HNSCC, and the comparison with other histopathological grading systems are insufficiently investigated.
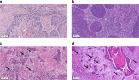
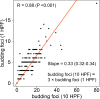
Methods: TB and MCNS were analysed digitally in 1 and 10 high-power fields (HPF) of 331 HPV-positive and HPV-negative cases from TCGA. Optimising the cutpoints a new cellular dissociation grading (CDG) system was defined and compared to the WHO grading and the Brandwein-Gensler (BG) risk model.
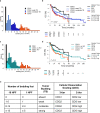
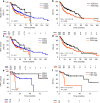
Results: The two-tiered CDG system based solely on TB yielded optimal prognostic stratification with shortened overall survival for CDG-high cases. Optimal cut-offs were two buds (1 HPF) and six buds (10 HPF), respectively. Analysing MCNS did not add prognostic significance to quantifying TB. CDG was a significant prognostic marker in HPV-negative and HPV-positive tumours and prognostically superior to the WHO and BG systems. High CDG was associated with clinically occult lymph-node metastases.
Conclusions: The most comprehensive study of TB in HNSCC so far confirmed its prognostic impact in HPV-negative tumours and for the first time in HPV-positive tumours. Further studies are warranted to evaluate its applicability for therapy guidance in HNSCC.
© 2023. The Author(s).
Conflict of interest statement
PS: Speaker’s fees: AstraZeneca, Incyte, Janssen. Advisory Boards: BMS, MSD, AstraZeneca, Roche. Funding for research: Roche, Chugai, BMS, Novartis. AS: Advisory Board/Speaker’s Bureau: Astra Zeneca, AGCT, Bayer, BMS, Eli Lilly, Illumina, Janssen, MSD, Novartis, Pfizer, Roche, Seattle Genetics, Takeda, Thermo Fisher. Grants: Bayer, BMS, Chugai, Incyte. WW: Advisory Boards and speaker’s fees: Roche, MSD, BMS, AstraZeneca, Pfizer, Merck, Lilly, Boehringer, Novartis, Takeda, Bayer, Amgen, Astellas, Eisai, Illumina, Siemens, Agilent, ADC, GSK and Molecular Health. Funding for research: Roche, MSD, BMS and AstraZeneca. MB: Funding through Deutsche Krebshilfe (German Cancer Aid). Advisory Board and speaker´s fee: BMS, MSD. Funding: BMS. JB: Funding through Deutsche Krebshilfe (German Cancer Aid). The remaining authors declare no competing interests.
Figures

Comment in
-
Tumour budding in head and neck cancer: what have we learnt and the next steps towards clinical implementation.Br J Cancer. 2024 Jan;130(1):1-2. doi: 10.1038/s41416-023-02531-4. Epub 2023 Dec 14. Br J Cancer. 2024. PMID: 38097743 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

