The little skate genome and the evolutionary emergence of wing-like fins
- PMID: 37046085
- PMCID: PMC10115646
- DOI: 10.1038/s41586-023-05868-1
The little skate genome and the evolutionary emergence of wing-like fins
Abstract
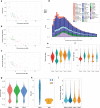
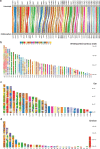
Skates are cartilaginous fish whose body plan features enlarged wing-like pectoral fins, enabling them to thrive in benthic environments1,2. However, the molecular underpinnings of this unique trait remain unclear. Here we investigate the origin of this phenotypic innovation by developing the little skate Leucoraja erinacea as a genomically enabled model. Analysis of a high-quality chromosome-scale genome sequence for the little skate shows that it preserves many ancestral jawed vertebrate features compared with other sequenced genomes, including numerous ancient microchromosomes. Combining genome comparisons with extensive regulatory datasets in developing fins-including gene expression, chromatin occupancy and three-dimensional conformation-we find skate-specific genomic rearrangements that alter the three-dimensional regulatory landscape of genes that are involved in the planar cell polarity pathway. Functional inhibition of planar cell polarity signalling resulted in a reduction in anterior fin size, confirming that this pathway is a major contributor to batoid fin morphology. We also identified a fin-specific enhancer that interacts with several hoxa genes, consistent with the redeployment of hox gene expression in anterior pectoral fins, and confirmed its potential to activate transcription in the anterior fin using zebrafish reporter assays. Our findings underscore the central role of genome reorganization and regulatory variation in the evolution of phenotypes, shedding light on the molecular origin of an enigmatic trait.
© 2023. The Author(s).
Conflict of interest statement
J.D. is on the scientific advisory board of Arima Genomics and of Omega Therapeutics. The other authors declare no competing interests.
Figures















Comment in
-
Genome reveals how the skate got its wings.Nature. 2023 Apr;616(7957):440-441. doi: 10.1038/d41586-023-00192-0. Nature. 2023. PMID: 37045951 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

