Extrachromosomal DNA in the cancerous transformation of Barrett's oesophagus
- PMID: 37046089
- PMCID: PMC10132967
- DOI: 10.1038/s41586-023-05937-5
Extrachromosomal DNA in the cancerous transformation of Barrett's oesophagus
Abstract
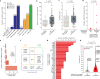
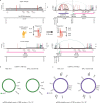
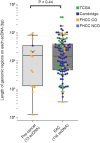
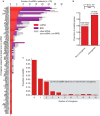
Oncogene amplification on extrachromosomal DNA (ecDNA) drives the evolution of tumours and their resistance to treatment, and is associated with poor outcomes for patients with cancer1-6. At present, it is unclear whether ecDNA is a later manifestation of genomic instability, or whether it can be an early event in the transition from dysplasia to cancer. Here, to better understand the development of ecDNA, we analysed whole-genome sequencing (WGS) data from patients with oesophageal adenocarcinoma (EAC) or Barrett's oesophagus. These data included 206 biopsies in Barrett's oesophagus surveillance and EAC cohorts from Cambridge University. We also analysed WGS and histology data from biopsies that were collected across multiple regions at 2 time points from 80 patients in a case-control study at the Fred Hutchinson Cancer Center. In the Cambridge cohorts, the frequency of ecDNA increased between Barrett's-oesophagus-associated early-stage (24%) and late-stage (43%) EAC, suggesting that ecDNA is formed during cancer progression. In the cohort from the Fred Hutchinson Cancer Center, 33% of patients who developed EAC had at least one oesophageal biopsy with ecDNA before or at the diagnosis of EAC. In biopsies that were collected before cancer diagnosis, higher levels of ecDNA were present in samples from patients who later developed EAC than in samples from those who did not. We found that ecDNAs contained diverse collections of oncogenes and immunomodulatory genes. Furthermore, ecDNAs showed increases in copy number and structural complexity at more advanced stages of disease. Our findings show that ecDNA can develop early in the transition from high-grade dysplasia to cancer, and that ecDNAs progressively form and evolve under positive selection.
© 2023. The Author(s).
Conflict of interest statement
P.S.M. is a co-founder, chairs the scientific advisory board (SAB) of and has equity interest in Boundless Bio. P.S.M. is also an advisor with equity for Asteroid Therapeutics and is an advisor to Sage Therapeutics. V.B. is a co-founder, consultant, SAB member and has equity interest in Boundless Bio and Abterra, and the terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. S.W. is a member of the SAB of Dimension Genomics. H.Y.C. is a co-founder of Accent Therapeutics, Boundless Bio, Cartography Bio, and Orbital Therapeutics, and is an advisor to 10X Genomics, Arsenal Biosciences, Chroma Medicine, and Spring Discovery. R.C.F. is named on patents related to Cytosponge and associated assays that were licensed to Covidien (now Medtronic). R.C.F. has founder shares (<3%) in Cyted. R.G.W.V. is a co-founder of Boundless Bio and an advisor to Stellanova Therapeutics and NeuroTrials. L.B.A. is a compensated consultant and has equity interest in io9. His spouse is an employee of Biotheranostics. L.B.A. is an inventor on the US patent 10,776,718 for source identification by non-negative matrix factorization. L.B.A. also declares provisional patent applications for ‘Clustered mutations for the treatment of cancer’ (US provisional application serial number 63/289,601) and ‘Artificial intelligence architecture for predicting cancer biomarker’ (serial number 63/269,033). S.M.L. is a co-founder of io9, and also declares a provisional patent application for ‘Methods and biomarkers in cancer’ (US provisional application serial number 114198-1160). J.L. is a part-time paid consultant for Boundless Bio, and the terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. P.S.M., V.B., J.L. and S.W. declare a patent application related to this work: ‘Methods and compositions for detecting ecDNA’ (US patent application number 17/746,748). The remaining authors declare no competing interests.
Figures









Comment in
-
Extrachromosomal DNA appears before cancer forms.Nature. 2023 Apr;616(7958):664-665. doi: 10.1038/d41586-023-00982-6. Nature. 2023. PMID: 37045953 No abstract available.

