Noncoding translation mitigation
- PMID: 37046090
- PMCID: PMC10560126
- DOI: 10.1038/s41586-023-05946-4
Noncoding translation mitigation
Abstract
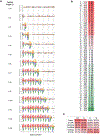
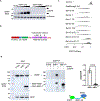
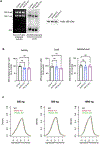
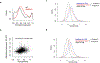
Translation is pervasive outside of canonical coding regions, occurring in long noncoding RNAs, canonical untranslated regions and introns1-4, especially in ageing4-6, neurodegeneration5,7 and cancer8-10. Notably, the majority of tumour-specific antigens are results of noncoding translation11-13. Although the resulting polypeptides are often nonfunctional, translation of noncoding regions is nonetheless necessary for the birth of new coding sequences14,15. The mechanisms underlying the surveillance of translation in diverse noncoding regions and how escaped polypeptides evolve new functions remain unclear10,16-19. Functional polypeptides derived from annotated noncoding sequences often localize to membranes20,21. Here we integrate massively parallel analyses of more than 10,000 human genomic sequences and millions of random sequences with genome-wide CRISPR screens, accompanied by in-depth genetic and biochemical characterizations. Our results show that the intrinsic nucleotide bias in the noncoding genome and in the genetic code frequently results in polypeptides with a hydrophobic C-terminal tail, which is captured by the ribosome-associated BAG6 membrane protein triage complex for either proteasomal degradation or membrane targeting. By contrast, canonical proteins have evolved to deplete C-terminal hydrophobic residues. Our results reveal a fail-safe mechanism for the surveillance of unwanted translation from diverse noncoding regions and suggest a possible biochemical route for the preferential membrane localization of newly evolved proteins.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
CONFLICT OF INTEREST
None.
Figures











Comment in
-
Noncoding translation: Quality control in the BAG.Mol Cell. 2023 Jun 15;83(12):1967-1969. doi: 10.1016/j.molcel.2023.05.033. Mol Cell. 2023. PMID: 37327774
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

