Antibodies against endogenous retroviruses promote lung cancer immunotherapy
- PMID: 37046094
- PMCID: PMC10115647
- DOI: 10.1038/s41586-023-05771-9
Antibodies against endogenous retroviruses promote lung cancer immunotherapy
Abstract
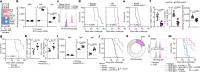
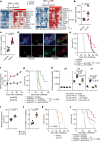
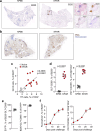
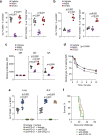
B cells are frequently found in the margins of solid tumours as organized follicles in ectopic lymphoid organs called tertiary lymphoid structures (TLS)1,2. Although TLS have been found to correlate with improved patient survival and response to immune checkpoint blockade (ICB), the underlying mechanisms of this association remain elusive1,2. Here we investigate lung-resident B cell responses in patients from the TRACERx 421 (Tracking Non-Small-Cell Lung Cancer Evolution Through Therapy) and other lung cancer cohorts, and in a recently established immunogenic mouse model for lung adenocarcinoma3. We find that both human and mouse lung adenocarcinomas elicit local germinal centre responses and tumour-binding antibodies, and further identify endogenous retrovirus (ERV) envelope glycoproteins as a dominant anti-tumour antibody target. ERV-targeting B cell responses are amplified by ICB in both humans and mice, and by targeted inhibition of KRAS(G12C) in the mouse model. ERV-reactive antibodies exert anti-tumour activity that extends survival in the mouse model, and ERV expression predicts the outcome of ICB in human lung adenocarcinoma. Finally, we find that effective immunotherapy in the mouse model requires CXCL13-dependent TLS formation. Conversely, therapeutic CXCL13 treatment potentiates anti-tumour immunity and synergizes with ICB. Our findings provide a possible mechanistic basis for the association of TLS with immunotherapy response.
Trial registration: ClinicalTrials.gov NCT01888601.
© 2023. The Author(s).
Conflict of interest statement
C.S. acknowledges grant support from AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc - collaboration in minimal residual disease sequencing technologies), Ono Pharmaceutical, and Personalis. He is an AstraZeneca Advisory Board member and Chief Investigator for the AZ MeRmaiD 1 and 2 clinical trials and is also Co-Chief Investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s Scientific Advisory Board. He receives consultant fees from Achilles Therapeutics (also SAB member), Bicycle Therapeutics (also a SAB member), Genentech, Medicxi, China Innovation Centre of Roche (CICoR) formerly Roche Innovation Centre – Shanghai, Metabomed (until July 2022), and the Sarah Cannon Research Institute C.S has received honoraria from Amgen, AstraZeneca, Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol Myers Squibb, Illumina, and Roche-Ventana. C.S. had stock options in Apogen Biotechnologies and GRAIL until June 2021, and currently has stock options in Epic Bioscience, Bicycle Therapeutics, and has stock options and is co-founder of Achilles Therapeutics. C.S. is an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289). The patents have been licensed to commercial entities, and under their terms of employment C.S. is due a revenue share of any revenue generated from such license(s). C.S. declares patent applications for targeting neoantigens (PCT/EP2016/059401), identifying patent response to ICB (PCT/EP2016/071471), determining HLA loss of heterozygosity (PCT/GB2018/052004), predicting survival rates of patients with cancer (PCT/GB2020/050221) and identifying patients who respond to cancer treatment (PCT/GB2018/051912); US patents relating to detecting tumour mutations (PCT/US2017/028013) and methods for lung cancer detection (US20190106751A1); and both a European and US patent related to identifying indel mutation targets (PCT/GB2018/051892) and is co-inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). G.K. is a scientific co-founder of EnaraBio and a member of its scientific advisory board. G.K. has consulted for EnaraBio and Repertoire Immune Medicines. J.D. has acted as a consultant for AstraZeneca, Bayer, Jubilant, Theras, BridgeBio, Vividion and Novartis and has funded research agreements with Bristol Myers Squibb, Revolution Medicines and AstraZeneca. K.S.S.E. acknowledges grant support from Bristol Myers Squibb. K.L. has a patent on indel burden and CPI response pending and has speaker fees from Roche tissue diagnostics, research funding from the CRUK TDL/Ono/LifeArc alliance and Genesis Therapeutics, and consulting roles with Monopteros Therapeutics and Kynos Therapeutics. J. Attig is currently an employee of and owns shares in Hoffmann-La Roche. S.V. is a co-inventor on a patent for detecting molecules in a sample (US patent 10578620). S.d.C.T. has acted as a consultant for Revolution Medicines. C.T.H. has received speaker fees from AstraZeneca. D.A.M. reports speaker fees from AstraZeneca, Eli Lilly and Takeda and consultancy fees from AstraZeneca, Thermo Fisher, Takeda, Amgen, Janssen, MIM Software, Bristol Myers Squibb and Eli Lilly and has received educational support from Takeda and Amgen. R.S. reports non-financial support from Merck and Bristol Myers Squibb, research support from Merck, Puma Biotechnology and Roche, and personal fees from Roche, Bristol Myers Squibb and Exact Sciences for advisory boards. A.M.F. is a co-inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). M.A.B. has consulted for Achilles Therapeutics. G.A.W. is employed by and has stock options in Achilles Therapeutics. N.J.B. is a co-inventor on a patent to identify responders to cancer treatment (PCT/GB2018/051912), has a patent application (PCT/GB2020/050221) on methods for cancer prognostication and is a co-inventor on a patent for methods for predicting anti-cancer response (US14/466,208). A.H. has received fees for being a member of the independent data monitoring committees for Roche-sponsored clinical trials and academic projects coordinated by Roche. N. McGranahan has received consultancy fees and has stock options in Achilles Therapeutics. N. McGranahan holds European patents relating to targeting neoantigens (PCT/EP2016/ 059401), identifying patient response to ICB (PCT/ EP2016/071471), determining HLA loss of heterozygosity (PCT/GB2018/052004) and predicting survival rates of patients with cancer (PCT/GB2020/050221). M.J.-H. has consulted for, and is a member of, the Achilles Therapeutics scientific advisory board and steering committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals and Oslo Cancer Cluster, and is a co-inventor on European patent application PCT/US2017/028013 relating to methods for lung cancer detection. This patent has been licensed to commercial entities, and under terms of employment M.J.-H. is due a share of any revenue generated from such license(s). The remaining authors declare no competing interests.
Figures








Comment in
-
Antibody-mediated immunity against lung cancer goes retro.Sci Immunol. 2023 Jun 8;8(84):eadi8769. doi: 10.1126/sciimmunol.adi8769. Epub 2023 Jun 2. Sci Immunol. 2023. PMID: 37276436
References
-
- Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science375, eabf9419 (2022). - PubMed
-
- Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med.381, 2020–2031 (2019). - PubMed
-
- Patil, N. S. et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell40, 289–300 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CC2097/WT_/Wellcome Trust/United Kingdom
- 21999/CRUK_/Cancer Research UK/United Kingdom
- CTRNBC-2022/100001/CRUK_/Cancer Research UK/United Kingdom
- 24956/CRUK_/Cancer Research UK/United Kingdom
- 17786/CRUK_/Cancer Research UK/United Kingdom
- 30025/CRUK_/Cancer Research UK/United Kingdom
- 29911/CRUK_/Cancer Research UK/United Kingdom
- CC2044/WT_/Wellcome Trust/United Kingdom
- MR/P014712/1/MRC_/Medical Research Council/United Kingdom
- CC2088/WT_/Wellcome Trust/United Kingdom
- CC2041/WT_/Wellcome Trust/United Kingdom
- MR/V033077/1/MRC_/Medical Research Council/United Kingdom
- 29569/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

