Investigating the Efficacy of HPV Vaccines in Preventing Cervical Cancer from 2006 to 2018 in the US: A SEER Data Set Analysis
- PMID: 37046192
- PMCID: PMC10514505
- DOI: 10.2174/1574887118666230410093715
Investigating the Efficacy of HPV Vaccines in Preventing Cervical Cancer from 2006 to 2018 in the US: A SEER Data Set Analysis
Abstract
Background: Human papillomavirus (HPV) is the most common sexually transmitted infection in the US.The first HPV vaccine was introduced in 2006. There are three different HPV vaccines that commonly target high-risk HPV types.
Objective: This study compares HPV vaccine efficacy based on alternative endpoints with the most recently available cervical cancer incidence data from the Surveillance, Epidemiology and End Results (SEER) program and SEER*Stat statistical software.
Methods: The incidence of cervical cancer, mined from the most recent April 2021 SEER data set, was stratified according to age and racial groups. Trend analysis reporting cervical cancer incidence percentage change (PC) and annual percentage change (APC) was calculated by SEER*Stat statistical software.
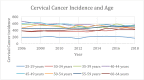
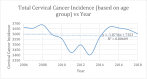
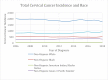
Results: A total of 46,583 cases of cervical cancer were reported, with an average of about 3,580 incidents of cervical cancer per year, with an overall decrement of about 60 cases over the period of 12 years. The percentage change according to age and race groups varied between -15.9 among 40- 44 years old (yo) and +13.8 among 30-34 yo, and from -12 among non-Hispanic White women to +13 among Hispanic women. Statistically significant APC was observed for five of the nine age groups and four of the five racial groups.
Conclusion: There seems to be little if any, correlation between cervical cancer incidence and the HPV vaccine program in the US. HPV vaccine efficacy based on alternative endpoints, such as nucleic acid testing and cytological, surgical, and seropositivity endpoints, is fair. Therefore, it is important to emphasize such alternative testing and surrogate endpoints.
Keywords: Human papillomavirus; SEER; alternative endpoints; cancer incidence; cervical cancer; sexually transmitted infection; vaccine efficacy.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The author declares no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Impact of human papillomavirus vaccine on cervical cancer epidemic: Evidence from the surveillance, epidemiology, and end results program.Front Public Health. 2023 Jan 6;10:998174. doi: 10.3389/fpubh.2022.998174. eCollection 2022. Front Public Health. 2023. PMID: 36684904 Free PMC article.
-
Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials.BMJ Open. 2017 Aug 18;7(8):e015867. doi: 10.1136/bmjopen-2017-015867. BMJ Open. 2017. PMID: 28821519 Free PMC article. Clinical Trial.
-
Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1-24. MMWR Recomm Rep. 2007. PMID: 17380109
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
-
Human papillomavirus vaccines: an update for gynecologists.Clin Obstet Gynecol. 2008 Sep;51(3):527-32. doi: 10.1097/GRF.0b013e31818092df. Clin Obstet Gynecol. 2008. PMID: 18677145 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

