Immunosuppressive therapy for progressive idiopathic membranous nephropathy: a cost-effectiveness analysis in China
- PMID: 37046255
- PMCID: PMC10091593
- DOI: 10.1186/s12913-023-09365-z
Immunosuppressive therapy for progressive idiopathic membranous nephropathy: a cost-effectiveness analysis in China
Abstract
Background: This study aims to evaluate the cost-effectiveness of immunosuppressive therapy for patients with progressive idiopathic membranous nephropathy (IMN) from the Chinese healthcare system perspective.
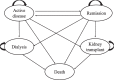
Methods: To estimate the cost-effectiveness of four regimens namely cyclophosphamide, cyclosporine, rituximab and tacrolimus-rituximab in treatment of IMN recommended by the updated Kidney Disease: Improving Global Outcomes (KDIGO) guideline 2021, a Markov model with five discrete states (active disease, remission, dialysis, kidney transplant and death) based on IMN patients aged 50 or above over a 30-years time horizon was constructed. Total costs were imputed from the Chinese healthcare system perspective, and health outcomes were converted into quality-adjusted life years (QALYs). The incremental cost-effectiveness ratio (ICER) was used to describe the results. The willingness-to-pay (WTP) threshold was set at $12,044 (China's 2021 Gross Domestic Product per capita). Sensitivity analyses were performed to test the uncertainties of the results.
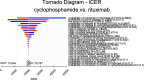
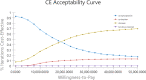
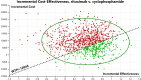
Result: Compared with cyclophosphamide, both cyclosporine (incremental cost $28,337.09, incremental QALY-1.63) and tacrolimus-rituximab (incremental cost $28,324.13, incremental QALY -0.46) were considered at strictly dominated for their negative values in QALYs, and the ICER value of rituximab was positive (incremental cost $9,162.19, incremental QALY 0.44). Since the ICER of rituximab exceeds the pre-determined threshold, cyclophosphamide was likely to be the best choice for the treatment of IMN within the acceptable threshold range. The results of the sensitivity analysis revealed that the model outcome was mostly affected by the probability of remission in rituximab. In a probabilistic sensitivity analysis, cyclophosphamide had 62.4% probability of being cost-effective compared with other regimens when the WTP was $12,044 per QALY. When WTP exceeded $18,300, rituximab was more cost-effective than cyclophosphamide.
Conclusion: Compared with cyclosporine, rituximab and tacrolimus-rituximab, our model results indicated that cyclophosphamide represented the most cost-effective regimen for patients with progressive IMN in China.
Keywords: Cost-effectiveness; Idiopathic membranous nephropathy; Immunosuppressive therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Is first-line treatment with polatuzumab vedotin-rituximab-cyclophosphamide, doxorubicin and prednisone (pola-R-CHP) for previously untreated diffuse large B-cell lymphoma cost-effective in China? A cost-effectiveness analysis using a Markov model.BMJ Open. 2025 Jan 20;15(1):e086251. doi: 10.1136/bmjopen-2024-086251. BMJ Open. 2025. PMID: 39832983 Free PMC article.
-
Rituximab or cyclosporine A for the treatment of membranous nephropathy: economic evaluation of the MENTOR trial.Nephrol Dial Transplant. 2024 Nov 27;39(12):2058-2066. doi: 10.1093/ndt/gfae084. Nephrol Dial Transplant. 2024. PMID: 38621719 Free PMC article. Clinical Trial.
-
Comparative pharmacoeconomic analysis of rituximab and traditional tacrolimus regimens in membranous nephropathy in China.Front Pharmacol. 2024 Jan 8;14:1309930. doi: 10.3389/fphar.2023.1309930. eCollection 2023. Front Pharmacol. 2024. PMID: 38259264 Free PMC article.
-
The economic impact associated with stent retriever selection for the treatment of acute ischemic stroke: a cost-effectiveness analysis of MASTRO I data from a Chinese healthcare system perspective.J Comp Eff Res. 2024 Nov;13(11):e240160. doi: 10.57264/cer-2024-0160. Epub 2024 Nov 5. J Comp Eff Res. 2024. PMID: 39498634 Free PMC article.
-
The clinical effectiveness and cost-effectiveness of rituximab for the first-line treatment of chronic lymphocytic leukaemia: an evidence review of the submission from Roche.Health Technol Assess. 2010 Oct;14(Suppl. 2):27-32. doi: 10.3310/hta14suppl2/04. Health Technol Assess. 2010. PMID: 21047488 Review.
Cited by
-
How to Choose the Right Treatment for Membranous Nephropathy.Medicina (Kaunas). 2023 Nov 14;59(11):1997. doi: 10.3390/medicina59111997. Medicina (Kaunas). 2023. PMID: 38004046 Free PMC article. Review.
References
-
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23(4):324–32.10.1016/s0270-9295(03)00049-4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

