Alpha-lipoic acid supplementation corrects pathological alterations in cellular models of pantothenate kinase-associated neurodegeneration with residual PANK2 expression levels
- PMID: 37046296
- PMCID: PMC10091671
- DOI: 10.1186/s13023-023-02687-5
Alpha-lipoic acid supplementation corrects pathological alterations in cellular models of pantothenate kinase-associated neurodegeneration with residual PANK2 expression levels
Abstract
Background: Neurodegeneration with brain iron accumulation (NBIA) disorders are a group of neurodegenerative diseases that have in common the accumulation of iron in the basal nuclei of the brain which are essential components of the extrapyramidal system. Frequent symptoms are progressive spasticity, dystonia, muscle rigidity, neuropsychiatric symptoms, and retinal degeneration or optic nerve atrophy. One of the most prevalent subtypes of NBIA is Pantothenate kinase-associated neurodegeneration (PKAN). It is caused by pathogenic variants in the gene of pantothenate kinase 2 (PANK2) which encodes the enzyme responsible for the first reaction on the coenzyme A (CoA) biosynthesis pathway. Thus, deficient PANK2 activity induces CoA deficiency as well as low expression levels of 4'-phosphopantetheinyl proteins which are essential for mitochondrial metabolism.
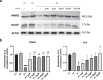
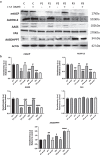
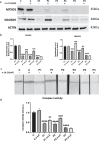
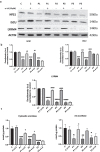
Methods: This study is aimed at evaluating the role of alpha-lipoic acid (α-LA) in reversing the pathological alterations in fibroblasts and induced neurons derived from PKAN patients. Iron accumulation, lipid peroxidation, transcript and protein expression levels of PANK2, mitochondrial ACP (mtACP), 4''-phosphopantetheinyl and lipoylated proteins, as well as pyruvate dehydrogenase (PDH) and Complex I activity were examined.
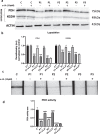
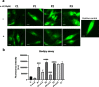
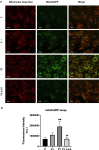
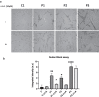
Results: Treatment with α-LA was able to correct all pathological alterations in responsive mutant fibroblasts with residual PANK2 enzyme expression. However, α-LA had no effect on mutant fibroblasts with truncated/incomplete protein expression. The positive effect of α-LA in particular pathogenic variants was also confirmed in induced neurons derived from mutant fibroblasts.
Conclusions: Our results suggest that α-LA treatment can increase the expression levels of PANK2 and reverse the mutant phenotype in PANK2 responsive pathogenic variants. The existence of residual enzyme expression in some affected individuals raises the possibility of treatment using high dose of α-LA.
Keywords: 4′-phosphopantetheinylation; Acyl carrier protein; Coenzyme A; Induced neurons; Mitochondria; PANK2; PKAN; Pantothenate kinase; Pantothenate kinase-associated neurodegeneration; α-lipoic acid.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Down regulation of the expression of mitochondrial phosphopantetheinyl-proteins in pantothenate kinase-associated neurodegeneration: pathophysiological consequences and therapeutic perspectives.Orphanet J Rare Dis. 2021 May 5;16(1):201. doi: 10.1186/s13023-021-01823-3. Orphanet J Rare Dis. 2021. PMID: 33952316 Free PMC article.
-
Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation.Mol Neurobiol. 2019 May;56(5):3638-3656. doi: 10.1007/s12035-018-1333-0. Epub 2018 Sep 1. Mol Neurobiol. 2019. PMID: 30173408
-
Therapeutic approach with commercial supplements for pantothenate kinase-associated neurodegeneration with residual PANK2 expression levels.Orphanet J Rare Dis. 2022 Aug 9;17(1):311. doi: 10.1186/s13023-022-02465-9. Orphanet J Rare Dis. 2022. PMID: 35945593 Free PMC article.
-
Patient-Derived Cellular Models for Polytarget Precision Medicine in Pantothenate Kinase-Associated Neurodegeneration.Pharmaceuticals (Basel). 2023 Sep 26;16(10):1359. doi: 10.3390/ph16101359. Pharmaceuticals (Basel). 2023. PMID: 37895830 Free PMC article. Review.
-
Unraveling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name.Curr Opin Pediatr. 2003 Dec;15(6):572-7. doi: 10.1097/00008480-200312000-00005. Curr Opin Pediatr. 2003. PMID: 14631201 Review.
Cited by
-
A Multi-Target Pharmacological Correction of a Lipoyltransferase LIPT1 Gene Mutation in Patient-Derived Cellular Models.Antioxidants (Basel). 2024 Aug 22;13(8):1023. doi: 10.3390/antiox13081023. Antioxidants (Basel). 2024. PMID: 39199267 Free PMC article.
-
Pathology and treatment methods in pantothenate kinase-associated neurodegeneration.Postep Psychiatr Neurol. 2024 Sep;33(3):163-171. doi: 10.5114/ppn.2024.141713. Epub 2024 Jul 23. Postep Psychiatr Neurol. 2024. PMID: 39678459 Free PMC article. Review.
-
Iron Accumulation and Lipid Peroxidation in Cellular Models of Nemaline Myopathies.Int J Mol Sci. 2025 Feb 8;26(4):1434. doi: 10.3390/ijms26041434. Int J Mol Sci. 2025. PMID: 40003902 Free PMC article.
-
Antioxidants Prevent Iron Accumulation and Lipid Peroxidation, but Do Not Correct Autophagy Dysfunction or Mitochondrial Bioenergetics in Cellular Models of BPAN.Int J Mol Sci. 2023 Sep 26;24(19):14576. doi: 10.3390/ijms241914576. Int J Mol Sci. 2023. PMID: 37834028 Free PMC article.
-
Polydatin and Nicotinamide Rescue the Cellular Phenotype of Mitochondrial Diseases by Mitochondrial Unfolded Protein Response (mtUPR) Activation.Biomolecules. 2024 May 18;14(5):598. doi: 10.3390/biom14050598. Biomolecules. 2024. PMID: 38786005 Free PMC article.
References
-
- Wilson JL, Gregory A, Kurian MA, Bushlin I, Mochel F, Emrick L, Adang L, Group BGCA, Hogarth P, Hayflick SJ: Consensus clinical management guideline for beta-propeller protein-associated neurodegeneration. Developmental medicine and child neurology 2021, 63(12):1402–1409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

