Adherence, helpfulness and barriers to treatment in juvenile idiopathic arthritis - data from a German Inception cohort
- PMID: 37046303
- PMCID: PMC10091650
- DOI: 10.1186/s12969-023-00811-0
Adherence, helpfulness and barriers to treatment in juvenile idiopathic arthritis - data from a German Inception cohort
Abstract
Objectives: To develop and evaluate German versions of the Parent Adherence Report Questionnaire (PARQ) and Child Adherence Report Questionnaire (CARQ) and to evaluate adherence in patients with juvenile idiopathic arthritis (JIA).
Methods: The PARQ and CARQ were translated into German, cross-culturally adapted and administered to patients (age ≥ 8 years) and their parents enrolled in the Inception Cohort Study of newly diagnosed JIA patients (ICON). The psychometric issues were explored by analyzing their test-retest reliability and construct validity.
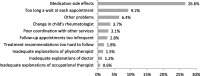
Results: Four hundred eighty-one parents and their children with JIA (n = 465) completed the PARQ and CARQ at the 4-year follow-up. Mean age and disease duration of patients were 10.1 ± 3.7 and 4.7 ± 0.8 years, respectively. The rate of missing values for PARQ/CARQ was generally satisfactory, test-retesting showed sufficient reliability. PARQ/CARQ mean child ability total scores (0-100, 100 = best) for medication were 73.1 ± 23.3/76.5 ± 24.2, for exercise: 85.6 ± 16.5/90.3 ± 15.0, for splints: 72.9 ± 24.2/82.9 ± 16.5. Construct validity was supported by PARQ and CARQ scores for medications, exercise and splints showing a fair to good correlation with the Global Adherence Assessment (GAA) and selected PedsQL scales. Adolescents showed poorer adherence than children. About one third of the parents and children reported medication errors. Perceived helpfulness was highest for medication, and adverse effects were reported the greatest barrier to treatment adherence.
Conclusions: The German versions of the PARQ and CARQ appear to have a good reliability and sufficient construct validity. These questionnaires are valuable tools for measuring treatment adherence, identifying potential barriers and evaluating helpfulness of treatments in patients with JIA.
Keywords: Adherence; Exercise; Juvenile idiopathic arthritis; Medication; Treatment.
© 2023. The Author(s).
Conflict of interest statement
SK: none; JK: none; IL: none; MN: none; DEF: none, FD: speaker`s fees from Novartis and Pfizer; IF: none, DF: none. JPH: none, GH: grants and personal fees from Abbvie, Chugai, MSD, Novartis, Pfizer, Roche, personal fees from Sobi, GSK, Sanofi, Bayer, AH: none, TK: none, JKD: none, KMm: none, FWH: none, DW: none, KM: received honoraria from Pfizer, Novartis and medac, CS: none.
Figures

References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
-
- Minden K, Kießling U, Listing J, Niewerth M, Döring E, Meincke J, et al. Prognosis of patients with juvenile chronic arthritis and juvenile spondylarthropathy. J Rheumatol. 2000;27(9):2256–2263. - PubMed
-
- Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29(9):1989–1999. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

