FAP-targeted CAR-T suppresses MDSCs recruitment to improve the antitumor efficacy of claudin18.2-targeted CAR-T against pancreatic cancer
- PMID: 37046312
- PMCID: PMC10091631
- DOI: 10.1186/s12967-023-04080-z
FAP-targeted CAR-T suppresses MDSCs recruitment to improve the antitumor efficacy of claudin18.2-targeted CAR-T against pancreatic cancer
Abstract
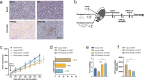
Purpose: The claudin 18.2 (CLDN18.2) antigen is frequently expressed in malignant tumors, including pancreatic ductal adenocarcinoma (PDAC). Although CLDN18.2-targeted CAR-T cells demonstrated some therapeutic efficacy in PDAC patients, further improvement is needed. One of the major obstacles might be the abundant cancer-associated fibroblasts (CAFs) in the PDAC tumor microenvironment (TME). Targeting fibroblast activation protein (FAP), a vital characteristic of CAFs provides a potential way to overcome this obstacle. In this study, we explored the combined antitumor activity of FAP-targeted and CLDN18.2-targeted CAR-T cells against PDAC.
Methods: Novel FAP-targeted CAR-T cells were developed. Sequential treatment of FAP-targeted and CLDN18.2-targeted CAR-T cells as well as the corresponding mechanism were explored in immunocompetent mouse models of PDAC.
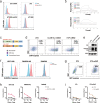
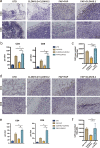
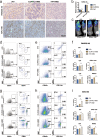
Results: The results indicated that the priorly FAP-targeted CAR-T cells infusion could significantly eliminate CAFs and enhance the anti-PDAC efficacy of subsequently CLDN18.2-targeted CAR-T cells in vivo. Interestingly, we observed that FAP-targeted CAR-T cells could suppress the recruitment of myeloid-derived suppressor cells (MDSCs) and promote the survival of CD8+ T cells and CAR-T cells in tumor tissue.
Conclusion: In summary, our finding demonstrated that FAP-targeted CAR-T cells could increase the antitumor activities of sequential CAR-T therapy via remodeling TME, at least partially through inhibiting MDSCs recruitment. Sequential infusion of FAP-targeted and CLDN18.2-targeted CAR-T cells might be a feasible approach to enhance the clinical outcome of PDAC.
© 2023. The Author(s).
Conflict of interest statement
Dr. Hua Jiang and Dr. Zonghai Li has ownership interests of CAR-T cells relating to this work and is a stockholder of CARsgen Therapeutics, Inc. The other authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Dual targeting chimeric antigen receptor cells enhance antitumour activity by overcoming T cell exhaustion in pancreatic cancer.Br J Pharmacol. 2024 Nov;181(22):4628-4646. doi: 10.1111/bph.16505. Epub 2024 Aug 11. Br J Pharmacol. 2024. PMID: 39129178
-
CXCR4-modified CAR-T cells suppresses MDSCs recruitment via STAT3/NF-κB/SDF-1α axis to enhance efficacy against pancreatic cancer.Mol Ther. 2023 Nov 1;31(11):3193-3209. doi: 10.1016/j.ymthe.2023.09.010. Epub 2023 Sep 20. Mol Ther. 2023. PMID: 37735875 Free PMC article.
-
Mesothelin CAR T Cells Secreting Anti-FAP/Anti-CD3 Molecules Efficiently Target Pancreatic Adenocarcinoma and its Stroma.Clin Cancer Res. 2024 May 1;30(9):1859-1877. doi: 10.1158/1078-0432.CCR-23-3841. Clin Cancer Res. 2024. PMID: 38393682 Free PMC article.
-
Research progress and design optimization of CAR-T therapy for pancreatic ductal adenocarcinoma.Cancer Med. 2019 Sep;8(11):5223-5231. doi: 10.1002/cam4.2430. Epub 2019 Jul 3. Cancer Med. 2019. PMID: 31339230 Free PMC article. Review.
-
Targeting fibroblast activation protein (FAP): advances in CAR-T cell, antibody, and vaccine in cancer immunotherapy.Drug Deliv Transl Res. 2023 Jul;13(7):2041-2056. doi: 10.1007/s13346-023-01308-9. Epub 2023 Feb 25. Drug Deliv Transl Res. 2023. PMID: 36840906 Review.
Cited by
-
CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn.Nat Rev Clin Oncol. 2024 Jan;21(1):47-66. doi: 10.1038/s41571-023-00832-4. Epub 2023 Oct 30. Nat Rev Clin Oncol. 2024. PMID: 37904019 Review.
-
Frontiers and future of immunotherapy for pancreatic cancer: from molecular mechanisms to clinical application.Front Immunol. 2024 May 2;15:1383978. doi: 10.3389/fimmu.2024.1383978. eCollection 2024. Front Immunol. 2024. PMID: 38756774 Free PMC article. Review.
-
Cancer associated fibroblasts in cancer development and therapy.J Hematol Oncol. 2025 Mar 28;18(1):36. doi: 10.1186/s13045-025-01688-0. J Hematol Oncol. 2025. PMID: 40156055 Free PMC article. Review.
-
Bibliometric analysis of global research trends in pancreatic cancer immunotherapy.Hum Vaccin Immunother. 2025 Dec;21(1):2538330. doi: 10.1080/21645515.2025.2538330. Epub 2025 Jul 24. Hum Vaccin Immunother. 2025. PMID: 40708169 Free PMC article.
-
Expression and Targeted Application of Claudins Family in Hepatobiliary and Pancreatic Diseases.J Hepatocell Carcinoma. 2024 Sep 25;11:1801-1821. doi: 10.2147/JHC.S483861. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 39345937 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

