Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma
- PMID: 37046332
- PMCID: PMC10091563
- DOI: 10.1186/s12964-023-01098-0
Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma
Abstract
Glioblastoma (GBM) is the most malignant CNS tumor with a highest incidence rate, and most patients would undergo a recurrence. Recurrent GBM (rGBM) shows an increasing resistance to chemotherapy and radiotherapy, leading to a significantly poorer prognosis and the urgent need for novel treatments. Immunotherapy, a rapidly developing anti-tumor therapy in recent years, has shown its potential value in rGBM. Recent studies on PD-1 immunotherapy and CAR-T therapy have shown some efficacy, but the outcome was not as expected. Tumor vaccination is the oldest approach of immunotherapies, which has returned to the research focus because of the failure of other strategies and subversive understanding of CNS. The isolation effect of blood brain barrier and the immunosuppressive cell infiltration could lead to resistance existing in all phases of the anti-tumor immune response, where novel tumor vaccines have been designed to overcome these problems through new tumor antigenic targets and regulatory of the systematic immune response. In this review, the immunological characteristics of CNS and GBM would be discussed and summarized, as well as the mechanism of each novel tumor vaccine for rGBM. And through the review of completed early-phase studies and ongoing large-scale phase III clinical trials, evaluation could be conducted for potential immune response, biosecurity and initial clinical outcome, which further draw a panorama of this vital research field and provide some deep thoughts for the prospective tendency of vaccination strategy. Video Abstract.
Keywords: Clinical trial; Immunotherapy; Recurrent glioblastoma; Tumor vaccine.
© 2023. The Author(s).
Conflict of interest statement
All of the authors declare that they have no competing interests or conflicts of interest.
Figures
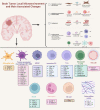


Similar articles
-
Recent developments in peptide vaccines against Glioblastoma, a review and update.Mol Brain. 2025 Jun 13;18(1):50. doi: 10.1186/s13041-025-01221-x. Mol Brain. 2025. PMID: 40514725 Free PMC article. Review.
-
Immunotherapy as a New Therapeutic Approach for Brain and Spinal Cord Tumors.Adv Exp Med Biol. 2023;1394:73-84. doi: 10.1007/978-3-031-14732-6_5. Adv Exp Med Biol. 2023. PMID: 36587382
-
Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds.Int Rev Immunol. 2022;41(6):582-605. doi: 10.1080/08830185.2022.2101647. Epub 2022 Aug 8. Int Rev Immunol. 2022. PMID: 35938932 Review.
-
Clinical Applications of Immunotherapy for Recurrent Glioblastoma in Adults.Cancers (Basel). 2023 Jul 31;15(15):3901. doi: 10.3390/cancers15153901. Cancers (Basel). 2023. PMID: 37568717 Free PMC article. Review.
-
An Update on the Role of Immunotherapy and Vaccine Strategies for Primary Brain Tumors.Curr Treat Options Oncol. 2015 Nov;16(11):54. doi: 10.1007/s11864-015-0371-3. Curr Treat Options Oncol. 2015. PMID: 26454859 Review.
Cited by
-
Targeting EGFR and PI3K/mTOR pathways in glioblastoma: innovative therapeutic approaches.Med Oncol. 2025 Mar 10;42(4):97. doi: 10.1007/s12032-025-02652-1. Med Oncol. 2025. PMID: 40064710 Review.
-
Recent developments in peptide vaccines against Glioblastoma, a review and update.Mol Brain. 2025 Jun 13;18(1):50. doi: 10.1186/s13041-025-01221-x. Mol Brain. 2025. PMID: 40514725 Free PMC article. Review.
-
Neuroinflammation in Glioblastoma: Progress and Perspectives.Brain Sci. 2024 Jul 9;14(7):687. doi: 10.3390/brainsci14070687. Brain Sci. 2024. PMID: 39061427 Free PMC article. Review.
-
Targeted immunotherapy for glioblastoma involving whole tumor-derived autologous cells in the upfront setting after craniotomy.J Neurooncol. 2023 Dec;165(3):389-398. doi: 10.1007/s11060-023-04491-4. Epub 2023 Nov 29. J Neurooncol. 2023. PMID: 38017340 Free PMC article. Review.
-
Targeting lymph node delivery with nanovaccines for cancer immunotherapy: recent advances and future directions.J Nanobiotechnology. 2023 Jul 7;21(1):212. doi: 10.1186/s12951-023-01977-1. J Nanobiotechnology. 2023. PMID: 37415161 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Board WHOWCoTE. Central nervous system tumours: WHO classification of tumours. International Agency for Research on Cancer (IARC); 2021.
-
- Berger TR, Wen PY, Lang-Orsini M, Chukwueke UN. World Health Organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. 2022;8(10):1493–1501. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

