Development of a micronucleus test using the EpiAirway™ organotypic human airway model
- PMID: 37046355
- PMCID: PMC10099928
- DOI: 10.1186/s41021-023-00269-2
Development of a micronucleus test using the EpiAirway™ organotypic human airway model
Abstract
Background: The use of organotypic human tissue models in genotoxicity has increased as an alternative to animal testing. Genotoxicity is generally examined using a battery of in vitro assays such as Ames and micronucleus (MN) tests that cover gene mutations and structural and numerical chromosome aberrations. At the 7th International Workshop on Genotoxicity Testing, working group members agreed that the skin models have reached an advanced stage of maturity, while further efforts in liver and airway models are needed [Pfuhler et al., Mutat. Res. 850-851 (2020) 503135]. Organotypic human airway model is composed of fully differentiated and functional respiratory epithelium. However, because cell proliferation in organotypic airway models is thought to be less active, assessing their MN-inducing potential is an issue, even in the cytokinesis-blocking approach using cytochalasin B (CB) [Wang et al., Environ. Mol. Mutagen. 62 (2021) 306-318]. Here, we developed a MN test using EpiAirway™ in which epidermal growth factor (EGF) was included as a stimulant of cell division.
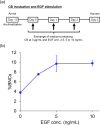
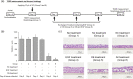
Results: By incubating EpiAirway™ tissue with medium containing various concentrations of CB, we found that the percentage of binucleated cells (%BNCs) almost plateaued at 3 μg/mL CB for 72 h incubation. Additionally, we confirmed that EGF stimulation with CB incubation produced an additional increase in %BNCs with a peak at 5 ng/mL EGF. Transepithelial electrical resistance measurement and tissue histology revealed that CB incubation caused the reduced barrier integrity and cyst formation in EpiAirway™. Adenylate kinase assay confirmed that the cytotoxicity increased with each day of culture in the CB incubation period with EGF stimulation. These results indicated that chemical treatment should be conducted prior to CB incubation. Under these experimental conditions, it was confirmed that the frequency of micronucleated cells was dose-dependently increased by apical applications of two clastogens, mitomycin C and methyl methanesulfonate, and an aneugen, colchicine, at the subcytotoxic concentrations assessed in %BNCs.
Conclusions: Well-studied genotoxicants demonstrated capability in an organotypic human airway model as a MN test system. For further utilization, investigations of aerosol exposure, repeating exposure protocol, and metabolic activation are required.
Keywords: Aneugen; Clastogens; EGF; EpiAirway™; Micronucleus test; Organotypic human airway model.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Insensitivity of the in vitro cytokinesis-block micronucleus assay with human lymphocytes for the detection of DNA damage present at the start of the cell culture.Mutagenesis. 2012 Nov;27(6):743-7. doi: 10.1093/mutage/ges041. Epub 2012 Aug 6. Mutagenesis. 2012. PMID: 22869611
-
Successful proof of concept of a micronucleus genotoxicity assay on reconstructed epidermis exhibiting intrinsic metabolic activity.Mutat Res Genet Toxicol Environ Mutagen. 2018 May-Jun;829-830:75-86. doi: 10.1016/j.mrgentox.2018.03.004. Epub 2018 Mar 10. Mutat Res Genet Toxicol Environ Mutagen. 2018. PMID: 29704997
-
High-throughput micronucleus assay using three-dimensional HepaRG spheroids for in vitro genotoxicity testing.Arch Toxicol. 2023 Apr;97(4):1163-1175. doi: 10.1007/s00204-023-03461-z. Epub 2023 Feb 27. Arch Toxicol. 2023. PMID: 36847820
-
Testing strategies in mutagenicity and genetic toxicology: an appraisal of the guidelines of the European Scientific Committee for Cosmetics and Non-Food Products for the evaluation of hair dyes.Mutat Res. 2005 Dec 30;588(2):88-105. doi: 10.1016/j.mrgentox.2005.09.006. Epub 2005 Dec 2. Mutat Res. 2005. PMID: 16326131 Review.
-
Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals?Mutagenesis. 2013 Jul;28(4):375-80. doi: 10.1093/mutage/get026. Epub 2013 May 3. Mutagenesis. 2013. PMID: 23644166 Review.
Cited by
-
A 3D Model of the Human Lung Airway for Evaluating Permeability of Inhaled Drugs.ACS Pharmacol Transl Sci. 2024 Dec 29;8(1):245-255. doi: 10.1021/acsptsci.4c00607. eCollection 2025 Jan 10. ACS Pharmacol Transl Sci. 2024. PMID: 39816797
-
Evaluation of Cytotoxicity and Oxidative Stress of Whole Aerosol from Vuse Alto ENDS Products.Toxics. 2024 Feb 4;12(2):129. doi: 10.3390/toxics12020129. Toxics. 2024. PMID: 38393224 Free PMC article.
-
Characterizing the Pulmonary Toxicity and Potential Mutagenicity of Formaldehyde Fumes in a Human Bronchial Epithelial Tissue Model.Environ Mol Mutagen. 2025 Jan-Feb;66(1-2):6-21. doi: 10.1002/em.70000. Epub 2025 Feb 24. Environ Mol Mutagen. 2025. PMID: 39991966
References
-
- Pridgeon CS, Schlott C, Wong MW, Heringa MB, Heckel T, Leedale J, et al. Innovative organotypic in vitro models for safety assessment: aligning with regulatory requirements and understanding models of the heart, skin, and liver as paradigms. Arch Toxicol. 2018;92(2):557–569. doi: 10.1007/s00204-018-2152-9. - DOI - PMC - PubMed
-
- Adriaens E, Barroso J, Eskes C, Hoffmann S, McNamee P, Alepee N, et al. Retrospective analysis of the Draize test for serious eye damage/eye irritation: importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Arch Toxicol. 2014;88(3):701–723. doi: 10.1007/s00204-013-1156-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

