Pre-Analytical Evaluation of Streck Cell-Free DNA Blood Collection Tubes for Liquid Profiling in Oncology
- PMID: 37046506
- PMCID: PMC10093569
- DOI: 10.3390/diagnostics13071288
Pre-Analytical Evaluation of Streck Cell-Free DNA Blood Collection Tubes for Liquid Profiling in Oncology
Abstract
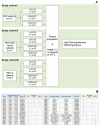
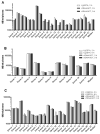
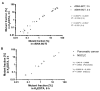
Excellent pre-analytical stability is an essential precondition for reliable molecular profiling of circulating tumor DNA (ctDNA) in oncological diagnostics. Therefore, in vitro degradation of ctDNA and the additional release of contaminating genomic DNA from lysed blood cells must be prevented. Streck Cell-Free DNA blood collection tubes (cfDNA BCTs) have proposed advantages over standard K2EDTA tubes, but mainly have been tested in healthy individuals. Blood was collected from cancer patients (n = 53) suffering from colorectal (n = 21), pancreatic (n = 11), and non-small-cell lung cancer (n = 21) using cfDNA BCT tubes and K2EDTA tubes that were processed immediately or after 3 days (BCTs) or 6 hours (K2EDTA) at room temperature. The cfDNA isolated from these samples was characterized in terms of yield using LINE-1 qPCR; the level of gDNA contamination; and the mutation status of KRAS, NRAS, and EGFR genes using BEAMing ddPCR. CfDNA yield and gDNA levels were comparable in both tube types and were not affected by prolonged storage of blood samples for at least 3 days in cfDNA BCTs or 6 hours in K2EDTA tubes. In addition, biospecimens collected in K2EDTA tubes and cfDNA BCTs stored for up to 3 days demonstrated highly comparable levels of mutational load across all respective cancer patient cohorts and a wide range of concentrations. Our data support the applicability of clinical oncology specimens collected and stored in cfDNA BCTs for up to 3 days for reliable cfDNA and mutation analyses.
Keywords: cell-free DNA (cfDNA); circulating tumor DNA (ctDNA); liquid profiling; pre-analytics; precision medicine.
Conflict of interest statement
I.M.D., A.N., F.D. and F.H. are employees of Sysmex Inostics GmbH. S.H. has received research funding and honoraria from Sysmex Inostics GmbH.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

