CDK4/6 Inhibition Induces Senescence and Enhances Radiation Response by Disabling DNA Damage Repair in Oral Cavity Squamous Cell Carcinoma
- PMID: 37046664
- PMCID: PMC10093103
- DOI: 10.3390/cancers15072005
CDK4/6 Inhibition Induces Senescence and Enhances Radiation Response by Disabling DNA Damage Repair in Oral Cavity Squamous Cell Carcinoma
Abstract
Purpose: HPV(-) OCSCC resists radiation treatment. The CDKN2A gene, encoding p16INK4A, is commonly disrupted in OCSCC. p16 inhibits CDK4/CDK6, leading to cell cycle arrest, but the biological sequelae of CDK4/6 inhibition in OCSCC remains understudied. This study examines whether inhibition of CDK4/6 enhances radiation response in OCSCC.
Methods: MTT assays were performed in OCSCC cell lines HN5 and CAL27 following treatment with palbociclib. Clonogenic survival and synergy were analyzed after radiation (RT-2 or 4Gy), palbociclib (P) (0.5 µM or 1 µM), or concurrent combination treatment (P+RT). DNA damage/repair and senescence were examined. CDK4/6 were targeted via siRNA to corroborate P+RT effects. Three-dimensional immortalized spheroids and organoids derived from patient tumors (conditionally reprogrammed OCSCC CR-06 and CR-18) were established to further examine and validate responses to P+RT.
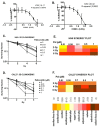
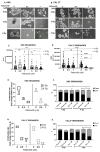
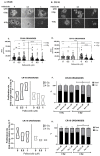
Results: P+RT demonstrated reduced viability and synergy, increased β-gal expression (~95%), and ~two-fold higher γH2AX. Rad51 and Ku80 were reduced after P+RT, indicating impairment of both HR and NHEJ. siCDK4/6 increased senescence with radiation. Spheroids showed reduced proliferation and size with P+RT. CR-06 and CR-18 further demonstrated three-fold reduced proliferation and organoids size with P+RT.
Conclusion: Targeting CDK4/6 can lead to improved efficacy when combined with radiation in OCSCC by inducing senescence and inhibiting DNA damage repair.
Keywords: CDK4; CDK6; DNA repair; PD-0332991 (palbociclib); head and neck squamous cell carcinoma (HNSCC); oral cavity; organoids; radiosensitizing; senescence.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Pignon J.P., Bourhis J., Domenge C., Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. doi: 10.1016/S0140-6736(00)90011-4. - DOI - PubMed
-
- Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. - DOI - PubMed
-
- Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C., et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

