Phase I Study of a Combination of Fluvastatin and Celecoxib in Children with Relapsing/Refractory Low-Grade or High-Grade Glioma (FLUVABREX)
- PMID: 37046681
- PMCID: PMC10093481
- DOI: 10.3390/cancers15072020
Phase I Study of a Combination of Fluvastatin and Celecoxib in Children with Relapsing/Refractory Low-Grade or High-Grade Glioma (FLUVABREX)
Abstract
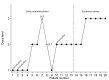
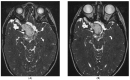
Preclinical data support the activity of celecoxib and fluvastatin in high-grade (HGG) and low-grade gliomas (LGG). A phase I trial (NCT02115074) was designed to evaluate the safety of this combination in children with refractory/relapsed HGG and LGG using four dose levels of fluvastatin with a fixed daily dose of celecoxib. A Continual Reassessment Method was used for fluvastatin dose escalation. Dose-limiting toxicities (DLT) were determined on the first treatment cycle. Twenty patients were included. Ten LGG and ten HGG patients received a median of 3.5 treatment cycles. Two DLTs were reported: one grade 3 maculopapular rash (4 mg/kg dose level) and one grade 4 increase of Creatine Phospho-Kinase (6 mg/kg dose level). We identified the dose of 6 mg/kg/day as the recommended phase II dose (RP2D) of fluvastatin with celecoxib. Four patients with LGG continued treatment beyond 12 cycles because of stable disease, including one patient who received 23 treatment cycles. In children with refractory/relapsed glioma, the RP2D of fluvastatin with celecoxib is 6 mg/kg/day. The long-term stable diseases observed in LGG suggest a possible role of the combination in a maintenance setting, given its good tolerance and low cost for children living in low- and middle-income countries.
Keywords: drug repurposing; high-grade pediatric glioma; low-grade pediatric glioma; phase I trial.
Conflict of interest statement
G.B., E.T.-B., M.-C.L.D., A.P., N.N., C.S., A.S., T.B., I.A., C.F.-C., A.-I.B., P.C., N.E.-W. and E.D.C. have no conflict of interest to declare. N.A. reports receiving grants and drugs for trials from Bristol Myers Squibb and Pierre Fabre Oncology, receiving travel support from Bristol Myers Squibb for an International Society of Paediatric Oncology meeting and participating as a scientific advisory board member (without receiving personal fees) for Bayer and Bristol Myers Squibb and Partners Therapeutics related to metronomic chemotherapy. P.L. reports receiving drugs for a clinical trial from Pierre Fabre Oncology, receiving drugs and grants for a clinical trial from Bristol Myers Squibb and participating as a scientific advisory board member (receiving personal fees) for AstraZeneca.
Figures


References
-
- Ostrom Q.T., de Blank P.M., Kruchko C., Petersen C.M., Liao P., Finlay J.L., Stearns D.S., Wolff J.E., Wolinsky Y., Letterio J.J., et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16((Suppl. 1)):x1–x36. doi: 10.1093/neuonc/nou327. - DOI - PMC - PubMed
-
- Bandopadhayay P., Bergthold G., London W.B., Goumnerova L.C., Morales La Madrid A., Marcus K.J., Guo D., Ullrich N.J., Robison N.J., Chi S.N., et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer. 2014;61:1173–1179. doi: 10.1002/pbc.24958. - DOI - PMC - PubMed
-
- Krishnatry R., Zhukova N., Guerreiro Stucklin A.S., Pole J.D., Mistry M., Fried I., Ramaswamy V., Bartels U., Huang A., Laperriere N., et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer. 2016;122:1261–1269. doi: 10.1002/cncr.29907. - DOI - PubMed
-
- Colin C., Padovani L., Chappé C., Mercurio S., Scavarda D., Loundou A., Frassineti F., Andre N., Bouvier C., Korshunov A., et al. Outcome analysis of childhood pilocytic astrocytomas: A retrospective study of 148 cases at a single institution. Neuropathol. Appl. Neurobiol. 2013;39:693–705. doi: 10.1111/nan.12013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical

