Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B
- PMID: 37046770
- PMCID: PMC10092996
- DOI: 10.3390/cancers15072109
Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B
Abstract
Background: Understanding the effect of selumetinib on FASI may help elucidate the biology, proliferative potential, and role in neurocognitive changes for these NF1-associated lesions.
Methods: Patients with NF1-associated LGG and FASI treated with selumetinib on PBTC-029B were age-matched to untreated patients with NF1-associated FASI at Cincinnati Children's Hospital Medical Center. Paired bidirectional measurements were compared over time using nonparametric tests.
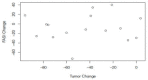
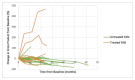
Results: Sixteen age-matched pairs were assessed (age range: 2.8-16.9 years, 60% male). Initial FASI burden was not different between groups (median range 138.7 cm2 [88.4-182.0] for the treated subjects vs. 121.6 cm2 [79.6-181.9] for the untreated subjects; p = 0.98). Over a mean follow-up of 18.9 (±5.9) months, the LGG size consistently decreased with treatment while no consistent change among the treated or untreated FASI size was seen. At the paired time points, the median treated LGG decreased significantly more than the treated FASI (-41.3% (LGG) versus -10.7% (FASI), p = 0.006). However, there was no difference in the median size change in the treated versus untreated FASI (-10.7% (treated FASI) versus -17.9% (untreated FASI), p = 0.08). Among the treated subjects, there was no correlation between the change in LGG and FASI (r = -0.04, p = 0.88).
Conclusions: Treatment with selumetinib did not affect the overall FASI size in children with NF1 treated for progressive low-grade glioma.
Keywords: FASI; MEK inhibitor; NF1; low grade glioma; selumetinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cerebellar radiological abnormalities in children with neurofibromatosis type 1: part 2 - a neuroimaging natural history study with clinical correlations.Cerebellum Ataxias. 2018 Oct 30;5:13. doi: 10.1186/s40673-018-0092-z. eCollection 2018. Cerebellum Ataxias. 2018. PMID: 30410778 Free PMC article.
-
Optic Pathway Glioma and Cerebral Focal Abnormal Signal Intensity in Patients with Neurofibromatosis Type 1: Characteristics, Treatment Choices and Follow-up in 134 Affected Individuals and a Brief Review of the Literature.Anticancer Res. 2016 Aug;36(8):4095-121. Anticancer Res. 2016. PMID: 27466519 Review.
-
A Phase 2 PBTC Study of Selumetinib for Recurrent/Progressive Pediatric Low-Grade Glioma: Strata 2, 5, and 6 with Long-term Outcomes on Strata 1, 3, and 4.Neuro Oncol. 2025 Apr 17:noaf065. doi: 10.1093/neuonc/noaf065. Online ahead of print. Neuro Oncol. 2025. PMID: 40241281
-
A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium study.Neuro Oncol. 2021 Oct 1;23(10):1777-1788. doi: 10.1093/neuonc/noab047. Neuro Oncol. 2021. PMID: 33631016 Free PMC article. Clinical Trial.
-
A Review of Selumetinib in the Treatment of Neurofibromatosis Type 1-Related Plexiform Neurofibromas.Ann Pharmacother. 2022 Jun;56(6):716-726. doi: 10.1177/10600280211046298. Epub 2021 Sep 18. Ann Pharmacother. 2022. PMID: 34541874 Review.
Cited by
-
Low-grade glioma in children with neurofibromatosis type 1: surveillance, treatment indications, management, and future directions.Childs Nerv Syst. 2024 Oct;40(10):3241-3250. doi: 10.1007/s00381-024-06430-8. Epub 2024 May 5. Childs Nerv Syst. 2024. PMID: 38704493 Review.
-
Multiparametric whole-body MRI of patients with neurofibromatosis type I: spectrum of imaging findings.Skeletal Radiol. 2025 Mar;54(3):407-422. doi: 10.1007/s00256-024-04765-6. Epub 2024 Aug 6. Skeletal Radiol. 2025. PMID: 39105762 Review.
References
-
- Fangusaro J., Onar-Thomas A., Young Poussaint T., Wu S., Ligon A.H., Lindeman N., Banerjee A., Packer R.J., Kilburn L.B., Goldman S., et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019;20:1011–1022. doi: 10.1016/S1470-2045(19)30277-3. - DOI - PMC - PubMed
-
- Salman M.S., Hossain S., Gorun S., Alqublan L., Bunge M., Rozovsky K. Cerebellar radiological abnormalities in children with neurofibromatosis type 1: Part 2—A neuroimaging natural history study with clinical correlations. Cerebellum Ataxias. 2018;5:13. doi: 10.1186/s40673-018-0092-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

