Potential, Limitations and Risks of Cannabis-Derived Products in Cancer Treatment
- PMID: 37046779
- PMCID: PMC10093248
- DOI: 10.3390/cancers15072119
Potential, Limitations and Risks of Cannabis-Derived Products in Cancer Treatment
Abstract
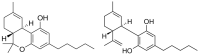
The application of cannabis products in oncology receives interest, especially from patients. Despite the plethora of research data available, the added value in curative or palliative cancer care and the possible risks involved are insufficiently proven and therefore a matter of debate. We aim to give a recommendation on the position of cannabis products in clinical oncology by assessing recent literature. Various types of cannabis products, characteristics, quality and pharmacology are discussed. Standardisation is essential for reliable and reproducible quality. The oromucosal/sublingual route of administration is preferred over inhalation and drinking tea. Cannabinoids may inhibit efflux transporters and drug-metabolising enzymes, possibly inducing pharmacokinetic interactions with anticancer drugs being substrates for these proteins. This may enhance the cytostatic effect and/or drug-related adverse effects. Reversely, it may enable dose reduction. Similar interactions are likely with drugs used for symptom management treating pain, nausea, vomiting and anorexia. Cannabis products are usually well tolerated and may improve the quality of life of patients with cancer (although not unambiguously proven). The combination with immunotherapy seems undesirable because of the immunosuppressive action of cannabinoids. Further clinical research is warranted to scientifically support (refraining from) using cannabis products in patients with cancer.
Keywords: cancer treatment; cannabidiol (CBD); cannabinoids; cannabis products; delta-9-tetrahydrocannabinol (THC); drug interactions; oncology; quality of life; symptom management.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lange B.M., Zager J.J. Comprehensive inventory of cannabinoids in Cannabis sativa L.: Can we connect genotype and chemotype? Phytochem. Rev. 2022;21:1273–1313. doi: 10.1007/s11101-021-09780-2. - DOI
-
- Plants of the World Online (Kew Royal Botanic Gardens) [(accessed on 2 November 2022)]. Available online: https://powo.science.kew.org.
Publication types
LinkOut - more resources
Full Text Sources