NMR-Metabolomics Reveals a Metabolic Shift after Surgical Resection of Non-Small Cell Lung Cancer
- PMID: 37046788
- PMCID: PMC10093525
- DOI: 10.3390/cancers15072127
NMR-Metabolomics Reveals a Metabolic Shift after Surgical Resection of Non-Small Cell Lung Cancer
Abstract
Background: Lung cancer can be detected by measuring the patient's plasma metabolomic profile using nuclear magnetic resonance (NMR) spectroscopy. This NMR-based plasma metabolomic profile is patient-specific and represents a snapshot of the patient's metabolite concentrations. The onset of non-small cell lung cancer (NSCLC) causes a change in the metabolite profile. However, the level of metabolic changes after complete NSCLC removal is currently unknown.
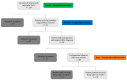
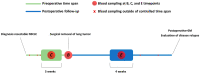
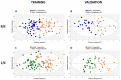
Patients and methods: Fasted pre- and postoperative plasma samples of 74 patients diagnosed with resectable stage I-IIIA NSCLC were analyzed using 1H-NMR spectroscopy. NMR spectra (s = 222) representing two preoperative and one postoperative plasma metabolite profile at three months after surgical resection were obtained for all patients. In total, 228 predictors, i.e., 228 variables representing plasma metabolite concentrations, were extracted from each NMR spectrum. Two types of supervised multivariate discriminant analyses were used to train classifiers presenting a strong differentiation between the pre- and postoperative plasma metabolite profiles. The validation of these trained classification models was obtained by using an independent dataset.
Results: A trained multivariate discriminant classification model shows a strong differentiation between the pre- and postoperative NSCLC profiles with a specificity of 96% (95% CI [86-100]) and a sensitivity of 92% (95% CI [81-98]). Validation of this model results in an excellent predictive accuracy of 90% (95% CI [77-97]) and an AUC value of 0.97 (95% CI [0.93-1]). The validation of a second trained model using an additional preoperative control sample dataset confirms the separation of the pre- and postoperative profiles with a predictive accuracy of 93% (95% CI [82-99]) and an AUC value of 0.97 (95% CI [0.93-1]). Metabolite analysis reveals significantly increased lactate, cysteine, asparagine and decreased acetate levels in the postoperative plasma metabolite profile.
Conclusions: The results of this paper demonstrate that surgical removal of NSCLC generates a detectable metabolic shift in blood plasma. The observed metabolic shift indicates that the NSCLC metabolite profile is determined by the tumor's presence rather than donor-specific features. Furthermore, the ability to detect the metabolic difference before and after surgical tumor resection strongly supports the prospect that NMR-generated metabolite profiles via blood samples advance towards early detection of NSCLC recurrence.
Keywords: NMR spectroscopy; metabolomics; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

