Perioperative Cetuximab with Cisplatin and 5-Fluorouracil in Esogastric Adenocarcinoma: A Phase II Study
- PMID: 37046849
- PMCID: PMC10093434
- DOI: 10.3390/cancers15072188
Perioperative Cetuximab with Cisplatin and 5-Fluorouracil in Esogastric Adenocarcinoma: A Phase II Study
Abstract
Purpose: While perioperative chemotherapy provides a survival benefit over surgery alone in gastric and gastroesophageal junction (G/GEJ) adenocarcinomas, the results need to be improved. This study aimed to evaluate the efficacy and safety of perioperative cetuximab combined with 5-fluorouracil and cisplatin.
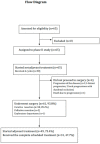
Patients and methods: Patients received six cycles of cetuximab, cisplatin, and simplified LV5FU2 before and after surgery. The primary objective was a combined evaluation of the tumor objective response (TOR), assessed by computed tomography, and the absence of major toxicities resulting in discontinuation of neoadjuvant chemotherapy (NCT) (45% and 90%, respectively).
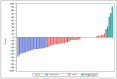
Results: From 2011 to 2013, 65 patients were enrolled. From 64 patients evaluable for the primary endpoint, 19 (29.7%) had a morphological TOR and 61 (95.3%) did not stop NCT prematurely due to major toxicity. Sixty patients (92.3%) underwent resection. Sixteen patients (/56 available, 28.5%) had histological responses (Mandard tumor regression grade ≤3). After a median follow-up of 44.5 months, median disease-free and overall survival were 24.4 [95% CI: 16.4-39.4] and 40.3 months [95% CI: 27.5-NA], respectively.
Conclusion: Adding cetuximab to the NCT regimen in operable G/GEJ adenocarcinomas is safe, but did not show enough efficacy in the present study to meet the primary endpoint (NCT01360086).
Keywords: EGFR-1; cetuximab; efficacy; esogastric adenocarcinoma; neoadjuvant chemotherapy; safety; surgery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zaanan A., Bouché O., Benhaim L., Buecher B., Chapelle N., Dubreuil O., Fares N., Granger V., Lefort C., Gagniere J., et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO) Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver. 2018;50:768–779. doi: 10.1016/j.dld.2018.04.025. - DOI - PubMed
-
- Ychou M., Boige V., Pignon J.-P., Conroy T., Bouché O., Lebreton G., Ducourtieux M., Bedenne L., Fabre J.-M., Saint-Aubert B., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

