Cholesterol Is a Regulator of CAV1 Localization and Cell Migration in Oral Squamous Cell Carcinoma
- PMID: 37047005
- PMCID: PMC10093846
- DOI: 10.3390/ijms24076035
Cholesterol Is a Regulator of CAV1 Localization and Cell Migration in Oral Squamous Cell Carcinoma
Abstract
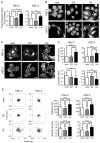
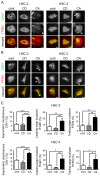
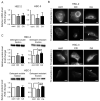
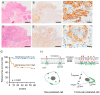
Cholesterol plays an important role in cancer progression, as it is utilized in membrane biogenesis and cell signaling. Cholesterol-lowering drugs have exhibited tumor-suppressive effects in oral squamous cell carcinoma (OSCC), suggesting that cholesterol is also essential in OSCC pathogenesis. However, the direct effects of cholesterol on OSCC cells remain unclear. Here, we investigated the role of cholesterol in OSCC with respect to caveolin-1 (CAV1), a cholesterol-binding protein involved in intracellular cholesterol transport. Cholesterol levels in OSCC cell lines were depleted using methyl-β-cyclodextrin and increased using the methyl-β-cyclodextrin-cholesterol complex. Functional analysis was performed using timelapse imaging, and CAV1 expression in cholesterol-manipulated cells was investigated using immunofluorescence and immunoblotting assays. CAV1 immunohistochemistry was performed on surgical OSCC samples. We observed that cholesterol addition induced polarized cell morphology, along with CAV1 localization at the trailing edge, and promoted cell migration. Moreover, CAV1 was upregulated in the lipid rafts and formed aggregates in the plasma membrane in cholesterol-added cells. High membranous CAV1 expression in tissue specimens was associated with OSCC recurrence. Therefore, cholesterol promotes the migration of OSCC cells by regulating cell polarity and CAV1 localization to the lipid raft. Furthermore, membranous CAV1 expression is a potential prognostic marker for OSCC patients.
Keywords: caveolin-1; cell polarization; cholesterol; migration; oral squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

