Synthesis and Anticancer Evaluation of 4-Anilinoquinolinylchalcone Derivatives
- PMID: 37047007
- PMCID: PMC10094048
- DOI: 10.3390/ijms24076034
Synthesis and Anticancer Evaluation of 4-Anilinoquinolinylchalcone Derivatives
Abstract
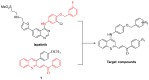
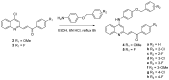
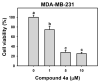
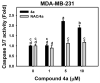
A series of 4-anilinoquinolinylchalcone derivatives were synthesized and evaluated for antiproliferative activities against the growth of human cancer cell lines (Huh-7 and MDA-MB-231) and normal lung cells (MRC-5). The results exhibited low cytotoxicity against human lung cells (MRC-5). Among them, (E)-3-{4-{[4-(benzyloxy)phenyl]amino}quinolin-2-yl}-1-(4-methoxyphenyl) prop-2-en-1-one (4a) was found to have the highest cytotoxicity in breast cancer cells and low cytotoxicity in normal cells. Compound 4a causes ATP depletion and apoptosis of breast cancer MDA-MB-231 cells and triggers reactive oxygen species (ROS)-dependent caspase 3/7 activation. In conclusion, it is worth studying 4-anilinoquinolinylchalcone derivatives further as new potential anticancer agents for the treatment of human cancers.
Keywords: 4-anilinoquinolinylchalcone; apoptosis; cytotoxicity; lapatinib; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

