Protein Arginine Methyltransferase 5 (PRMT5) Mutations in Cancer Cells
- PMID: 37047013
- PMCID: PMC10094674
- DOI: 10.3390/ijms24076042
Protein Arginine Methyltransferase 5 (PRMT5) Mutations in Cancer Cells
Abstract
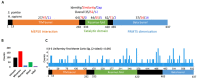
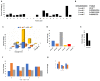
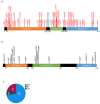
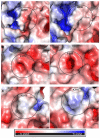
Arginine methylation is a form of posttranslational modification that regulates many cellular functions such as development, DNA damage repair, inflammatory response, splicing, and signal transduction, among others. Protein arginine methyltransferase 5 (PRMT5) is one of nine identified methyltransferases, and it can methylate both histone and non-histone targets. It has pleiotropic functions, including recruitment of repair machinery to a chromosomal DNA double strand break (DSB) and coordinating the interplay between repair and checkpoint activation. Thus, PRMT5 has been actively studied as a cancer treatment target, and small molecule inhibitors of its enzymatic activity have already been developed. In this report, we analyzed all reported PRMT5 mutations appearing in cancer cells using data from the Catalogue of Somatic Mutations in Cancers (COSMIC). Our goal is to classify mutations as either drivers or passengers to understand which ones are likely to promote cellular transformation. Using gold standard artificial intelligence algorithms, we uncovered several key driver mutations in the active site of the enzyme (D306H, L315P, and N318K). In silico protein modeling shows that these mutations may affect the affinity of PRMT5 for S-adenosylmethionine (SAM), which is required as a methyl donor. Electrostatic analysis of the enzyme active site shows that one of these mutations creates a tunnel in the vicinity of the SAM binding site, which may allow interfering molecules to enter the enzyme active site and decrease its activity. We also identified several non-coding mutations that appear to affect PRMT5 splicing. Our analyses provide insights into the role of PRMT5 mutations in cancer cells. Additionally, since PRMT5 single molecule inhibitors have already been developed, this work may uncover future directions in how mutations can affect targeted inhibition.
Keywords: arginine methylation; cancer; mutation; post-translational modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Histone H2A and H4 N-terminal tails are positioned by the MEP50 WD repeat protein for efficient methylation by the PRMT5 arginine methyltransferase.J Biol Chem. 2015 Apr 10;290(15):9674-89. doi: 10.1074/jbc.M115.636894. Epub 2015 Feb 24. J Biol Chem. 2015. PMID: 25713080 Free PMC article.
-
PRMT5-Dependent Methylation of the TIP60 Coactivator RUVBL1 Is a Key Regulator of Homologous Recombination.Mol Cell. 2017 Mar 2;65(5):900-916.e7. doi: 10.1016/j.molcel.2017.01.019. Epub 2017 Feb 23. Mol Cell. 2017. PMID: 28238654 Free PMC article.
-
The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond.Cell Mol Life Sci. 2015 Jun;72(11):2041-59. doi: 10.1007/s00018-015-1847-9. Epub 2015 Feb 7. Cell Mol Life Sci. 2015. PMID: 25662273 Free PMC article. Review.
-
A TGFβ-PRMT5-MEP50 axis regulates cancer cell invasion through histone H3 and H4 arginine methylation coupled transcriptional activation and repression.Oncogene. 2017 Jan 19;36(3):373-386. doi: 10.1038/onc.2016.205. Epub 2016 Jun 6. Oncogene. 2017. PMID: 27270440 Free PMC article.
-
Role of protein arginine methyltransferase 5 in human cancers.Biomed Pharmacother. 2019 Jun;114:108790. doi: 10.1016/j.biopha.2019.108790. Epub 2019 Mar 20. Biomed Pharmacother. 2019. PMID: 30903920 Review.
Cited by
-
Genetic Alterations of NF-κB and Its Regulators: A Rich Platform to Advance Colorectal Cancer Diagnosis and Treatment.Int J Mol Sci. 2023 Dec 21;25(1):154. doi: 10.3390/ijms25010154. Int J Mol Sci. 2023. PMID: 38203325 Free PMC article. Review.
-
Regulation of DNA repair gene expression by PRMT5.MicroPubl Biol. 2025 Jul 14;2025:10.17912/micropub.biology.001631. doi: 10.17912/micropub.biology.001631. eCollection 2025. MicroPubl Biol. 2025. PMID: 40735501 Free PMC article.
-
Therapeutic targeting of protein arginine methyltransferases reduces breast cancer progression by disrupting angiogenic pathways.Biochem Biophys Rep. 2025 Jul 31;43:102172. doi: 10.1016/j.bbrep.2025.102172. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40791817 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

