Molecular Landscape of Pelvic Organ Prolapse Provides Insights into Disease Etiology
- PMID: 37047060
- PMCID: PMC10094264
- DOI: 10.3390/ijms24076087
Molecular Landscape of Pelvic Organ Prolapse Provides Insights into Disease Etiology
Abstract
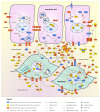
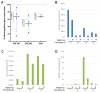
Pelvic organ prolapse (POP) represents a major health care burden in women, but its underlying pathophysiological mechanisms have not been elucidated. We first used a case-control design to perform an exome chip study in 526 women with POP and 960 control women to identify single nucleotide variants (SNVs) associated with the disease. We then integrated the functional interactions between the POP candidate proteins derived from the exome chip study and other POP candidate molecules into a molecular landscape. We found significant associations between POP and SNVs in 54 genes. The proteins encoded by 26 of these genes fit into the molecular landscape, together with 43 other POP candidate molecules. The POP landscape is located in and around epithelial cells and fibroblasts of the urogenital tract and harbors four interacting biological processes-epithelial-mesenchymal transition, immune response, modulation of the extracellular matrix, and fibroblast function-that are regulated by sex hormones and TGFB1. Our findings were corroborated by enrichment analyses of differential gene expression data from an independent POP cohort. Lastly, based on the landscape and using vaginal fibroblasts from women with POP, we predicted and showed that metformin alters gene expression in these fibroblasts in a beneficial direction. In conclusion, our integrated molecular landscape of POP provides insights into the biological processes underlying the disease and clues towards novel treatments.
Keywords: TGFB1; exome chip study; genetics; metformin; pelvic organ prolapse; single nucleotide variants (SNVs).
Conflict of interest statement
GP is the director, and JW as well as WDW are employees of Drug Target ID, Ltd., but their activities at this company do not constitute competing interests with regard to this paper. All other authors declare they have no competing interests.
Figures
Similar articles
-
Comparative Characterization of Vaginal Cells Derived From Premenopausal Women With and Without Severe Pelvic Organ Prolapse.Reprod Sci. 2016 Jul;23(7):931-43. doi: 10.1177/1933719115625840. Epub 2016 Jan 13. Reprod Sci. 2016. PMID: 26763525
-
Differential gene expression of extracellular-matrix-related proteins in the vaginal apical compartment of women with pelvic organ prolapse.Int Urogynecol J. 2019 Mar;30(3):439-446. doi: 10.1007/s00192-018-3637-z. Epub 2018 Mar 29. Int Urogynecol J. 2019. PMID: 29600404
-
Expression of ArfGAP3 in Vaginal Anterior Wall of Patients With Pelvic Floor Organ Prolapse in Pelvic Organ Prolapse and Non-Pelvic Organ Prolapse Patients.Female Pelvic Med Reconstr Surg. 2021 Jan 1;27(1):e64-e69. doi: 10.1097/SPV.0000000000000808. Female Pelvic Med Reconstr Surg. 2021. PMID: 31868832 Free PMC article.
-
Genetic Etiology in Pelvic Organ Prolapse: Role of Connective Tissue Homeostasis, Hormone Metabolism, and Oxidative Stress.Genes (Basel). 2024 Dec 24;16(1):5. doi: 10.3390/genes16010005. Genes (Basel). 2024. PMID: 39858552 Free PMC article. Review.
-
Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse.Biomolecules. 2022 Jan 6;12(1):94. doi: 10.3390/biom12010094. Biomolecules. 2022. PMID: 35053242 Free PMC article. Review.
Cited by
-
Identifying Therapeutic Targets for Amyotrophic Lateral Sclerosis Through Modeling of Multi-Omics Data.Int J Mol Sci. 2025 Jul 23;26(15):7087. doi: 10.3390/ijms26157087. Int J Mol Sci. 2025. PMID: 40806220 Free PMC article.
-
Cytokine modulation in pelvic organ prolapse and urinary incontinence: from molecular insights to therapeutic targets.Mol Med. 2024 Nov 13;30(1):214. doi: 10.1186/s10020-024-00989-3. Mol Med. 2024. PMID: 39538179 Free PMC article. Review.
-
Developing a polygenic risk score for pelvic organ prolapse: a combined risk assessment approach in Chinese women.Front Med. 2025 Aug;19(4):665-674. doi: 10.1007/s11684-024-1114-2. Epub 2025 May 3. Front Med. 2025. PMID: 40317452
-
Effect of Functionalization of Texturized Polypropylene Surface by Silanization and HBII-RGD Attachment on Response of Primary Abdominal and Vaginal Fibroblasts.Polymers (Basel). 2024 Feb 29;16(5):667. doi: 10.3390/polym16050667. Polymers (Basel). 2024. PMID: 38475352 Free PMC article.
References
-
- Slieker-ten Hove M.C., Pool-Goudzwaard A.L., Eijkemans M.J., Steegers-Theunissen R.P., Burger C.W., Vierhout M.E. The prevalence of pelvic organ prolapse symptoms and signs and their relation with bladder and bowel disorders in a general female population. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2009;20:1037–1045. doi: 10.1007/s00192-009-0902-1. - DOI - PMC - PubMed
-
- Schulten S.F.M., Claas-Quax M.J., Weemhoff M., van Eijndhoven H.W., van Leijsen S.A., Vergeldt T.F., IntHout J., Kluivers K.B. Risk factors for primary pelvic organ prolapse and prolapse recurrence: An updated systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022;227:192–208. doi: 10.1016/j.ajog.2022.04.046. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

