Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps
- PMID: 37047067
- PMCID: PMC10094365
- DOI: 10.3390/ijms24076094
Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps
Abstract
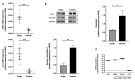
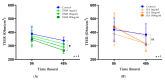
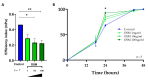
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a typical type-2 inflammation involving several cytokines and is associated with epithelial cell dysfunction. Oncostatin M (OSM) (belonging to the interleukin(IL)-6 family) could be a key driver of epithelial barrier dysfunction. Therefore, we investigated the presence of OSM and IL-6 and the expression pattern of tight junctions (TJs) in the nasal tissue of CRSwNP patients and controls using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) and Western blotting. Then, their potential role in the epithelial barrier was evaluated in vitro in 27 different primary cultures of human nasal epithelial cells (HNECs) by measuring TJ expression and transepithelial electric resistance (TEER) with or without OSM or IL-6 (1, 10, and 100 ng/mL). The effect on ciliary beating efficiency was evaluated by high-speed videomicroscopy and on repair mechanisms with a wound healing model with or without OSM. OSM and IL-6 were both overexpressed, and TJ (ZO-1 and occludin) expression was decreased in the nasal polyps compared to the control mucosa. OSM (100 ng/mL) but not IL-6 induced a significant decrease in TJ expression, TEER, and ciliary beating efficiency in HNECs. After 24 h, the wound repair rate was significantly higher in OSM-stimulated HNECs at 100 ng/mL. These results suggest that OSM could become a new target for monoclonal antibodies.
Keywords: CRSwNP; IL-6; OSM; ciliary beating efficiency; epithelial electric resistance; nasal epithelium; primary culture; repair rate; tight junctions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
H3N2 influenza virus infection enhances oncostatin M expression in human nasal epithelium.Exp Cell Res. 2018 Oct 15;371(2):322-329. doi: 10.1016/j.yexcr.2018.08.022. Epub 2018 Aug 22. Exp Cell Res. 2018. PMID: 30142324
-
Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease.J Allergy Clin Immunol. 2015 Sep;136(3):737-746.e4. doi: 10.1016/j.jaci.2015.01.043. Epub 2015 Apr 1. J Allergy Clin Immunol. 2015. PMID: 25840724 Free PMC article.
-
Evidence that oncostatin M synergizes with IL-4 signaling to induce TSLP expression in chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2023 May;151(5):1379-1390.e11. doi: 10.1016/j.jaci.2022.11.029. Epub 2023 Jan 6. J Allergy Clin Immunol. 2023. PMID: 36623776 Free PMC article.
-
Updated epithelial barrier dysfunction in chronic rhinosinusitis: Targeting pathophysiology and treatment response of tight junctions.Allergy. 2024 May;79(5):1146-1165. doi: 10.1111/all.16064. Epub 2024 Feb 19. Allergy. 2024. PMID: 38372149 Review.
-
[The role of epithelial cells in the formation and development of nasal polyps].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Nov;34(11):1053-1056. doi: 10.13201/j.issn.2096-7993.2020.11.024. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 33254333 Free PMC article. Review. Chinese.
Cited by
-
The Direct and Indirect Role of IgE on Airway Epithelium in Asthma.Allergy. 2025 Apr;80(4):919-931. doi: 10.1111/all.16459. Epub 2025 Feb 18. Allergy. 2025. PMID: 39963805 Free PMC article. Review.
-
IL-4Rα signaling promotes barrier-altering oncostatin M and IL-6 production in aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2024 Aug;154(2):458-467.e3. doi: 10.1016/j.jaci.2024.04.020. Epub 2024 May 3. J Allergy Clin Immunol. 2024. PMID: 38704098 Free PMC article.
References
-
- Tomassen P., Vandeplas G., van Zele T., Cardell L.-O., Arebro J., Olze H., Förster-Ruhrmann U., Kowalski M.L., Olszewska-Ziąber A., Holtappels G., et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. - DOI - PubMed
-
- Soyka M.B., Wawrzyniak P., Eiwegger T., Holzmann D., Treis A., Wanke K., Kast J.I., Akdis C.A. Defective Epithelial Barrier in Chronic Rhinosinusitis: The Regulation of Tight Junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012;130:1087–1096.e10. doi: 10.1016/j.jaci.2012.05.052. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

