Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide
- PMID: 37047079
- PMCID: PMC10094148
- DOI: 10.3390/ijms24076107
Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide
Abstract
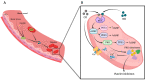
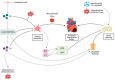
Nitric oxide (NO) and carbon monoxide (CO) represent a pair of biologically active gases with an increasingly well-defined range of effects on circulating platelets. These gases interact with platelets and cells in the vessels and heart and exert fundamentally similar biological effects, albeit through different mechanisms and with some peculiarity. Within the cardiovascular system, for example, the gases are predominantly vasodilators and exert antiaggregatory effects, and are protective against damage in myocardial ischemia-reperfusion injury. Indeed, NO is an important vasodilator acting on vascular smooth muscle and is able to inhibit platelet activation. NO reacts with superoxide anion (O2(-•)) to form peroxynitrite (ONOO(-)), a nitrosating agent capable of inducing oxidative/nitrative signaling and stress both at cardiovascular, platelet, and plasma levels. CO reduces platelet reactivity, therefore it is an anticoagulant, but it also has some cardioprotective and procoagulant properties. This review article summarizes current knowledge on the platelets and roles of gas mediators (NO, and CO) in cardioprotection. In particular, we aim to examine the link and interactions between platelets, NO, and CO and cardioprotective pathways.
Keywords: gasotransmitters; nitrosating agents; platelets; preconditioning; remote conditioning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Davidson S.M., Adameová A., Barile L., Cabrera-Fuentes H.A., Lazou A., Pagliaro P., Stensløkken K.-O., Garcia-Dorado D. EU-Cardioprotection Cost Action (CA16225) Mitochondrial and Mitochondrial-Independent Pathways of Myocardial Cell Death during Ischaemia and Reperfusion Injury. J. Cell. Mol. Med. 2020;24:3795–3806. doi: 10.1111/jcmm.15127. - DOI - PMC - PubMed

