Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus
- PMID: 37047117
- PMCID: PMC10093849
- DOI: 10.3390/ijms24076144
Low P-Selectin Glycoprotein Ligand-1 Expression in Neutrophils Associates with Disease Activity and Deregulated NET Formation in Systemic Lupus Erythematosus
Abstract
Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by the generation of anti-DNA autoantibodies due to exposure of immune cells to excessive amounts of extracellular DNA. Lack of P-selectin in mice induces the development of a lupus-like syndrome and patients with cutaneous lupus have reduced P-selectin expression in skin vessels. Using flow cytometry we analyzed in healthy donors and patients the expression of P-selectin Glycoprotein Ligand-1 (PSGL-1) in circulating neutrophils and the implication of PSGL-1/P-selectin interaction in neutrophil extracellular traps (NETs) generation. We found a statistical significance that neutrophils from active SLE patients have a reduced expression of PSGL-1 and low levels of PSGL-1 in neutrophils from SLE patients associated with the presence of anti-dsDNA antibodies, clinical lung involvement, Raynaud's phenomenon, and positive lupus anticoagulant. PSGL-1 is present along the DNA in the NET. In healthy donors, neutrophil interaction with immobilized P-selectin triggers Syk activation, increases the NETs percentage and reduces the amount of DNA extruded in the NETs. In active SLE patients, neutrophil interaction with P-selectin does not activate Syk or reduce the amount of DNA extruded in the NETs, that might contribute to increase the extracellular level of DNA and hence, to disease pathogenesis.
Keywords: NETs; P-selectin; PSGL-1; SLE pathogenesis; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

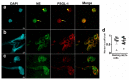
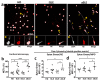
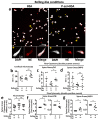


References
MeSH terms
Substances
Grants and funding
- FIS-PI17-01819/Spanish Ministry of Health and ISCIII (co-financed by Fondos FEDER)
- FIS-PI20-01690/Spanish Ministry of Health and ISCIII (co-financed by Fondos FEDER)
- EuroNanoMed III AC17-0027/Spanish Ministry of Health and ISCIII (co-financed by Fondos FEDER)
- "Molecular stratification of patients with giant cell arteritis to tailor glucocorticoid and tocili-zumab therapy (START Project)"/FOREUM, Foundation for Research in Rheumatology
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

