Molecular Docking Identifies 1,8-Cineole (Eucalyptol) as A Novel PPARγ Agonist That Alleviates Colon Inflammation
- PMID: 37047133
- PMCID: PMC10094723
- DOI: 10.3390/ijms24076160
Molecular Docking Identifies 1,8-Cineole (Eucalyptol) as A Novel PPARγ Agonist That Alleviates Colon Inflammation
Abstract
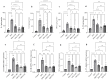
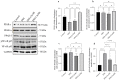
Inflammatory bowel disease, comprising Crohn's disease (CD) and ulcerative colitis (UC), is often debilitating. The disease etiology is multifactorial, involving genetic susceptibility, microbial dysregulation, abnormal immune activation, and environmental factors. Currently, available drug therapies are associated with adverse effects when used long-term. Therefore, the search for new drug candidates to treat IBD is imperative. The peroxisome proliferator-activated receptor-γ (PPARγ) is highly expressed in the colon. PPARγ plays a vital role in regulating colonic inflammation. 1,8-cineole, also known as eucalyptol, is a monoterpene oxide present in various aromatic plants which possess potent anti-inflammatory activity. Molecular docking and dynamics studies revealed that 1,8-cineole binds to PPARγ and if it were an agonist, that would explain the anti-inflammatory effects of 1,8-cineole. Therefore, we investigated the role of 1,8-cineole in colonic inflammation, using both in vivo and in vitro experimental approaches. Dextran sodium sulfate (DSS)-induced colitis was used as the in vivo model, and tumor necrosis factor-α (TNFα)-stimulated HT-29 cells as the in vitro model. 1,8-cineole treatment significantly decreased the inflammatory response in DSS-induced colitis mice. 1,8-cineole treatment also increased nuclear factor erythroid 2-related factor 2 (Nrf2) translocation into the nucleus to induce potent antioxidant effects. 1,8-cineole also increased colonic PPARγ protein expression. Similarly, 1,8-cineole decreased proinflammatory chemokine production and increased PPARγ protein expression in TNFα-stimulated HT-29 cells. 1,8-cineole also increased PPARγ promoter activity time-dependently. Because of its potent anti-inflammatory effects, 1,8-cineole may be valuable in treating IBD.
Keywords: 1,8-cineole (eucalyptol); DSS-colitis; IBD; PPARγ.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Molecular Docking, Dynamics, and Preclinical Studies Reveal Morin Hydrate as a Potent PPARγ and Nrf2 Agonist That Mitigates Colon Inflammation.Pharmacol Res Perspect. 2025 Aug;13(4):e70132. doi: 10.1002/prp2.70132. Pharmacol Res Perspect. 2025. PMID: 40611672 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Oroxyloside prevents dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB pathway through PPARγ activation.Biochem Pharmacol. 2016 Apr 15;106:70-81. doi: 10.1016/j.bcp.2016.02.019. Epub 2016 Mar 4. Biochem Pharmacol. 2016. PMID: 26947454
-
A Novel Topical PPARγ Agonist Induces PPARγ Activity in Ulcerative Colitis Mucosa and Prevents and Reverses Inflammation in Induced Colitis Models.Inflamm Bowel Dis. 2018 Mar 19;24(4):792-805. doi: 10.1093/ibd/izx079. Inflamm Bowel Dis. 2018. PMID: 29529198
-
Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor γ in inflammatory bowel diseases.Phytother Res. 2020 Jul;34(7):1530-1549. doi: 10.1002/ptr.6625. Epub 2020 Feb 3. Phytother Res. 2020. PMID: 32009281 Review.
Cited by
-
Research advancements and perspectives of inflammatory bowel disease: A comprehensive review.Sci Prog. 2024 Apr-Jun;107(2):368504241253709. doi: 10.1177/00368504241253709. Sci Prog. 2024. PMID: 38778725 Free PMC article. Review.
-
Plant Monoterpenes and Essential Oils as Potential Anti-Ageing Agents: Insights from Preclinical Data.Biomedicines. 2024 Feb 4;12(2):365. doi: 10.3390/biomedicines12020365. Biomedicines. 2024. PMID: 38397967 Free PMC article. Review.
-
Rhanterium Epapposum Essential Oil and Its Primary Compounds Control Infection, Inflammation, and Serum Electrolyte Imbalance in Mice with Giardiasis.Acta Parasitol. 2025 Jan 24;70(1):37. doi: 10.1007/s11686-024-00974-0. Acta Parasitol. 2025. PMID: 39853617
-
Terpenes: Modulating anti-inflammatory signaling in inflammatory bowel disease.Pharmacol Ther. 2023 Aug;248:108456. doi: 10.1016/j.pharmthera.2023.108456. Epub 2023 May 27. Pharmacol Ther. 2023. PMID: 37247693 Free PMC article. Review.
-
Effect of Callistemon citrinus Phytosomes on Oxidative Stress in the Brains of Rats Fed a High-Fat-Fructose Diet.Biomolecules. 2025 Aug 5;15(8):1129. doi: 10.3390/biom15081129. Biomolecules. 2025. PMID: 40867573 Free PMC article.
References
-
- McDowell C., Farooq U., Haseeb M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 1 January 2023)]. Inflammatory bowel disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ - PubMed
-
- Decara J., Rivera P., López-Gambero A.J., Serrano A., Pavón F.J., Baixeras E., De Fonseca F.R., Suárez J. Peroxisome proliferator-activated receptors: Experimental targeting for the treatment of inflammatory bowel diseases. Front. Pharmacol. 2020;11:730. doi: 10.3389/fphar.2020.00730. - DOI - PMC - PubMed
-
- Krönke G., Kadl A., Ikonomu E., Blü;ml S., Fürnkranz A., Sarembock I.J., Bochkov V.N., Exner M., Binder B.R., Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arter. Thromb. Vasc. Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. - DOI - PubMed

